- Record: found
- Abstract: found
- Article: found
SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19?
article-commentary
Gabriela M Kuster
e1
,
e2 ,
Otmar Pfister
e1
,
e2 ,
Thilo Burkard
e1
,
e3 ,
Qian Zhou
e1 ,
Raphael Twerenbold
e1
,
e4 ,
Philip Haaf
e1 ,
Andreas F Widmer
e5 ,
Stefan Osswald
e1
20 March 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
In a rapid response published online by the British Medical Journal, Sommerstein and
Gräni
1
pushed forward the hypothesis that angiotensin-converting enzyme (ACE) inhibitors
(ACE-Is) could act as a potential risk factor for fatal Corona virus disease 2019
(COVID-19) by up-regulating ACE2. This notion was quickly picked up by the lay press
and sparked concerns among physicians and patients regarding the intake of inhibitors
of the renin–angiotensin–aldosterone system (RAAS) by severe acute respiratory syndrome
coronavirus 2 (SARS-CoV2) infected individuals.
1
In this article, we try to shed light on what is known and unknown regarding the RAAS
and SARS-CoV2 interaction. We find translational evidence for diverse roles of the
RAAS, which allows to formulate also the opposite hypothesis, i.e. that inhibition
of the RAAS might be protective in COVID-19.
As of March 11, 124 910 patients worldwide have been tested positive for COVID-19
with a reported death toll amounting to 4589 patients, and the numbers continue to
rise.
2
First analyses of patient characteristics from China showed that diabetes, hypertension,
and cardiovascular diseases are highly prevalent among SARS-CoV2 infected patients,
and may be associated with poor outcome.
3
Specifically, their prevalence was roughly three- to four-fold increased among patients
reaching the combined primary endpoint of admission to an intensive care unit, mechanical
ventilation, or death compared to patients with less severe outcomes. In general,
patients with these conditions are frequently treated with inhibitors of the RAAS,
namely ACE-Is, angiotensin II type 1 receptor blockers (ARBs), or mineralocorticoid
receptor antagonists (MRAs).
As previously shown for SARS-CoV,
4
SARS-CoV2
5
similarly utilizes ACE2 as receptor for viral cell entry. In the RAAS, ACE2 catalyses
the conversion of angiotensin II to angiotensin 1–7, which acts as a vasodilator and
exerts protective effects in the cardiovascular system. In animal experiments, increased
expression and activity of ACE2 in various organs including the heart were found in
connection with ACE-I and ARB administration.
6
In addition, more recent data showing increased urinary secretion of ACE2 in hypertensive
patients treated with the ARB olmesartan suggest that up-regulation of ACE2 may also
occur in humans.
7
These observations have been reiterated in the literature and on the web in recent
days and the question arose whether RAAS inhibition may increase the risk of deleterious
outcome of COVID-19 through up-regulation of ACE2 and increase of viral load.
Despite the possible up-regulation of ACE2 by RAAS inhibition and the theoretically
associated risk of a higher susceptibility to infection, there is currently no data
proving a causal relationship between ACE2 activity and SARS-CoV2 associated mortality.
Furthermore, ACE2 expression may not necessarily correlate with the degree of infection.
Although ACE2 is thought to be mandatory for SARS-CoV infection, absence of SARS-CoV
was observed in some ACE2 expressing cell types, whereas infection was present in
cells apparently lacking ACE2, suggesting that additional co-factors might be needed
for efficient cellular infection.
8
In addition, lethal outcome of COVID-19 is mostly driven by the severity of the underlying
lung injury. Importantly, in a mouse model of SARS-CoV infection and pulmonary disease,
a key pathophysiological role was shown for ACE, angiotensin II and angiotensin II
receptor type 1.
9
SARS-CoV or SARS-CoV spike protein led to down-regulation of ACE2 and more severe
lung injury in mice that could be attenuated by administration of an ARB
9
,
10
These findings suggest a protective role of ARB in SARS-CoV associated lung injury
and give rise to the hypothesis that primary activation of the RAAS in cardiovascular
patients, rather than its inhibition, renders them more prone to a deleterious outcome.
11
It is important to note that Guan et al.
3
do not report how many patients were taking ACE-Is or ARBs. Based on data from the
China PEACE Million Persons Project, nearly half of Chinese adults between 35 and
75 years are suffering from hypertension, but fewer than one third receive treatment,
and blood pressure control is achieved in less than 10%.
12
Furthermore, there is thus far no data showing that hypertension or diabetes are independent
predictors of fatal outcome. Therefore, based on currently available data and statistics,
the assumption of a causal relationship between ACE-I or ARB intake and deleterious
outcome in COVID-19 is not legitimate. In fact, in a case of reverse causality, patients
taking ACE-Is or ARBs may be more susceptible for viral infection and have higher
mortality because they are older, more frequently hypertensive, diabetic, and/or having
renal disease.
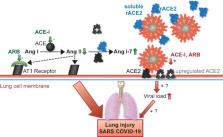
Take home figure
Conceptual figure highlighting the central role of ACE2 in the potentially deleterious
(red) and protective (green) effects of the RAAS and its inhibition in the development
of severe acute respiratory syndrome (SARS). ACE-Is and ARBs increase ACE2 expression
and activity (grey) as shown by a few animal and human studies,
6
,
7
but the mechanism has yet to be identified. Although there is currently no evidence,
this could theoretically increase viral load and worsen outcome (red). In a reverse
causality, ACE2 acts as a gatekeeper of the RAAS by degrading AngII to Ang1-7, hence
diminishing its Ang II receptor 1-mediated deleterious effects. Therefore, ACE-I or
ARB treatment could theoretically mitigate lung injury (green). Evidence for this
mainly stems from animal studies.
9
,
10
Providing soluble recombinant (r)ACE2 (blue) addresses both mechanisms by cell independent
binding of SARS-CoV2 and degrading AngII to Ang 1-7. This concept is currently being
tested in a pilot study in patients with COVID-19.
13
Clearly, much more research is needed to clarify the multifaceted role of the RAAS
in connection with SARS-CoV2 infection. Although there is data from animal studies
suggesting potentially deleterious effects of the RAAS, prove-of-concept in humans
is still lacking. Similarly, a few animal and human studies suggest up-regulation
of ACE2 in response to RAAS inhibition through a yet to be identified mechanism, but
whether this increases viral load in a critical way, and how viral load per se relates
to disease severity remains unknown. Nevertheless, based on the work by Josef Penninger
et al.,
13
who proposed to therapeutically use the dual function of ACE2 as viral receptor and
gatekeeper of RAAS activation, a pilot trial using soluble human recombinant ACE2
(APN01) in patients with COVID-19 has recently been initiated (Clinicaltrials.gov
#NCT04287686). Such therapy could have the potential to lower both the viral load
and the deleterious effects of angiotensin II activity.
In the meantime, we are well-advised to stick to what is known. There is abundant
and solid evidence of the mortality-lowering effects of RAAS inhibitors in cardiovascular
disease. ACE-Is, ARBs, and MRAs are the cornerstone of a prognostically beneficial
heart failure therapy with the highest level of evidence with regard to mortality
reduction.
14
They all have in common the inhibition of the adverse cardiovascular effects arising
from the interaction of angiotensin II with the angiotensin II receptor type 1. Discontinuation
of heart failure therapy leads to deterioration of cardiac function and heart failure
within days to weeks with a possible respective increase in mortality.
15–17
Similarly, ACE-Is, ARBs, and MRAs are part of the standard therapy in hypertension
18
and after myocardial infarction.
19
Significant reduction of post-infarct mortality applies to all three substance classes,
whereby early initiation of therapy (within days after infarction) is an important
factor of success.
20–23
In conclusion, based on currently available data and in view of the overwhelming evidence
of mortality reduction in cardiovascular disease, ACE-I and ARB therapy should be
maintained or initiated in patients with heart failure, hypertension, or myocardial
infarction according to current guidelines as tolerated, irrespective of SARS-CoV2.
Withdrawal of RAAS inhibition or preemptive switch to alternate drugs at this point
seems not advisable, since it might even increase cardiovascular mortality in critically
ill COVID-19 patients.
Conflict of interest: O.P. reports personal fees from Novartis, personal fees from
Pfizer, grants and personal fees from Boehringer Ingelheim, grants and personal fees
from AstraZeneca, grants from Sanofi, personal fees from Vifor Pharma, personal fees
from MSD, outside the submitted work. T.B. reports personal fees from Servier, Amgen,
Takeda, Menarini, MSD, Sanofi, and Vifor, outside the submitted work. R.T. reports
personal fees from Abbott, Amgen, Astra Zeneca, Roche Diagnostics, Siemens, Singulex,
and Thermo Scientific BRAHMS, outside the submitted work. Q.Z. reports grants from
Boehringer Ingelheim, personal fees from Astra Zeneca, grants from Abbott, personal
fees from Novartis, other from Alnylam, and personal fees from Bayer, outside the
submitted work. S.O. reports grants from the Swiss National Science Foundation for
the SwissAF cohort study, outside the submitted work. All other authors declared no
conflict of interest.
Related collections
Most cited references13
- Record: found
- Abstract: found
- Article: not found
Clinical Characteristics of Coronavirus Disease 2019 in China
Wei-jie Guan, Zheng-yi Ni, Yu Hu … (2020)
- Record: found
- Abstract: found
- Article: not found
An interactive web-based dashboard to track COVID-19 in real time
Ensheng Dong, Hongru Du, Lauren Gardner (2020)

- Record: found
- Abstract: found
- Article: found
Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target
Haibo Zhang, Josef M. Penninger, Yimin Li … (2020)