- Record: found
- Abstract: found
- Article: found
Protocol for a partially nested randomized controlled trial to evaluate the effectiveness of the Scleroderma Patient-centered Intervention Network Support Group Leader EDucation (SPIN-SSLED) Program
Read this article at
Abstract
Background
Some people with rare diseases rely on peer-led support groups for disease-specific education and emotional and practical support. Systemic sclerosis (SSc), or scleroderma, is a rare autoimmune connective tissue disease. Many people with SSc cannot access support groups, and, when support groups exist, they may not be sustained due to challenges that could be addressed via leader training. The Scleroderma Patient-centered Intervention Network (SPIN), along with SSc patient organization partners, developed a training program for SSc patient support group leaders, the Scleroderma Support group Leader EDucation (SPIN-SSLED) Program. We recently completed a feasibility trial in which we successfully delivered the program to two groups of support group leaders who reported a high level of satisfaction with the program and its delivery. The primary objective of the full-scale SPIN-SSLED trial is to evaluate the effect of the program on support group leaders’ self-efficacy for carrying out their leadership role. Secondary objectives include evaluating effects on leader burnout, leader satisfaction (participation efficacy), and emotional distress.
Methods/design
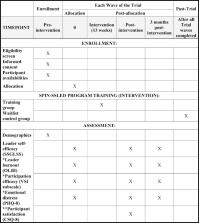
The SPIN-SSLED trial is a pragmatic randomized controlled trial (RCT) in which 180 support group leaders will be randomly allocated to training groups of 6 participants each or to a waitlist control. We will use a partially nested RCT design to reflect dependence between individuals in training groups, but not in the waitlist control. Participants allocated to the training program will receive the 13-module SPIN-SSLED Program, delivered via webinar over the course of 3 months in weekly 60–90-min sessions. The primary outcome is leader self-efficacy, measured by the Scleroderma Support Group Leader Self-efficacy Scale post-intervention. Secondary outcomes are leader self-efficacy at 3 months post-intervention, and leader burnout, volunteer job satisfaction (participation efficacy), and emotional distress post-intervention and at 3 months post-intervention.
Discussion
The SPIN-SSLED trial will test whether a training program for SSc patient support group leaders increases the self-efficacy of group leaders to carry out leadership tasks. The program has the potential to significantly improve the effectiveness and sustainability of existing SSc support groups, to increase the number of available support groups, and to be adapted for other chronic diseases.
Related collections
Most cited references56
- Record: found
- Abstract: not found
- Article: not found
The PRECIS-2 tool: designing trials that are fit for purpose.
- Record: found
- Abstract: not found
- Article: not found
Problem-Based Learning: What and How Do Students Learn?
- Record: found
- Abstract: found
- Article: not found