- Record: found
- Abstract: found
- Article: found
Priming With Recombinant BCG Expressing Novel HIV-1 Conserved Mosaic Immunogens and Boosting With Recombinant ChAdOx1 Is Safe, Stable, and Elicits HIV-1-Specific T-Cell Responses in BALB/c Mice
Read this article at
Abstract
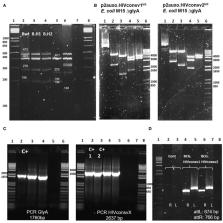
BCG is currently the only licensed vaccine against tuberculosis (TB) and confers protection against meningitis and miliary tuberculosis in infants, although pulmonary disease protection in adults is inconsistent. Recently, promising HIV-1 immunogens were developed, such as the T-cell immunogens “tHIVconsvX,” designed using functionally conserved protein regions across group M strains, with mosaic immunogens to improve HIV-1 variant match and response breadth. In this study, we constructed an integrative E. coli-mycobacterial shuttle plasmid, p2auxo.HIVconsvX int, expressing the immunogens HIVconsv1&2. This expression vector used an antibiotic resistance-free mechanism for plasmid selection and maintenance. It was first transformed into a glycine auxotrophic E. coli strain and subsequently transformed into a lysine auxotrophic Mycobacterium bovis BCG strain to generate vaccines BCG.HIVconsv1 2auxo.int and BCG.HIVconsv2 2auxo.int. The DNA sequence coding for the HIVconsv1&2 immunogens and protein expression were confirmed and working vaccine stocks were genetically and phenotypically characterized. We demonstrated that BCG.HIVconsv1&2 2auxo.int in combination with ChAdOx1.tHIVconsv5&6 were well tolerated and induced HIV-1-specific T-cell responses in adult BALB/c mice. In addition, we showed that the BCG.HIVconsv1&2 2auxo.int vaccine strains were stable in vitro after 35 bacterial generations and in vivo 7 weeks after inoculation. The use of integrative expression vectors and novel HIV-1 immunogens are likely to have improved the mycobacterial vaccine stability and specific immunogenicity and may enable the development of a useful vaccine platform for priming protective responses against HIV-1/TB and other prevalent pediatric pathogens shortly following birth.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome.
- Record: found
- Abstract: found
- Article: not found
Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors.
- Record: found
- Abstract: found
- Article: not found