- Record: found
- Abstract: found
- Article: not found
Immune-relevant aspects of murine models of head and neck cancer
Abstract
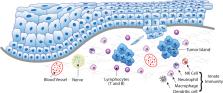
Head and neck cancers (HNC) cause significant mortality and morbidity. There have been few advances in therapeutic management of HNC in the past 4 to 5 decades, which supports the need for studies focusing on HNC biology. In recent years, increased recognition of the relevance of the host response in cancer progression has led to novel therapeutic strategies and putative biomarkers of tumor aggressiveness. However, tumor-immune interactions are highly complex and vary with cancer type. Pre-clinical, in vivo models represent an important and necessary step in understanding biological processes involved in development, progression and treatment of HNC. Rodents (mice, rats, hamsters) are the most frequently used animal models in HNC research. The relevance and utility of information generated by studies in murine models is unquestionable, but it is also limited in application to tumor-immune interactions. In this review, we present information regarding the immune-specific characteristics of the murine models most commonly used in HNC research, including immunocompromised and immunocompetent animals. The particular characteristics of xenograft, chemically-induced, syngeneic, transgenic, and humanized models are discussed in order to provide context and insight for researchers interested in the in vivo study of tumor-immune interactions in HNC.
Related collections
Most cited references157
- Record: found
- Abstract: found
- Article: not found
The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway.
- Record: found
- Abstract: found
- Article: not found
Inflammation: gearing the journey to cancer.
- Record: found
- Abstract: found
- Article: not found
