- Record: found
- Abstract: found
- Article: found
The impact of Anastrazole and Letrozole on the metabolic profile in an experimental animal model
Read this article at
Abstract
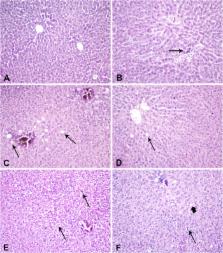
Anastrazole and Letrozole are used as endocrine therapy for breast cancer patients. Previous studies suggested a possible association with metabolic and liver adverse effects. Their results are conflicting. Fifty-five 4-week-old female Wistar rats were allocated in 4 groups 1) ovariectomy control (OC), 2) ovariectomy-Anastrazole (OA) 3) ovariectomy -Letrozole (OL), 4) control. Serum glucose, cholesterol, triglycerides, HDL-c and LDL-c were measured at baseline, 2 and 4 months. At the end, the animals‘ liver were dissected for pathology. At 4 months, total cholesterol differed among the OC and OL groups ( p = 0.15) and the control and OL groups ( p = 0.12). LDL-C differed between the control and OC groups ( p = 0.015) as well as between the control and OA ( p =0 .015) and OL groups ( p = 0.002). OC group triglycerides, differed from those of the OL group ( p =0 .002) and the control group ( p = 0.007). The OA also significantly differed from the OL ( p = 0.50). Liver pathology analysis revealed differences among groups with favored mild steatosis and ballooning. Anastrazole and Letrozole seem to negatively influence the lipid profile in our experimental model. This information should be taken in caution by medical oncologists when addressing patients with altered lipid metabolism.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer.
- Record: found
- Abstract: found
- Article: not found
Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis.
- Record: found
- Abstract: found
- Article: not found