- Record: found
- Abstract: found
- Article: found
Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections

Read this article at
SUMMARY
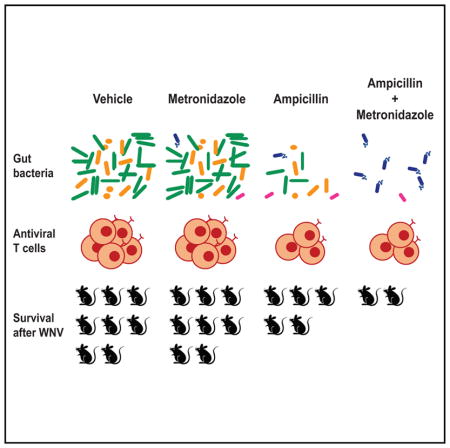
Although the outcome of flavivirus infection can vary from asymptomatic to lethal, environmental factors modulating disease severity are poorly defined. Here, we observed increased susceptibility of mice to severe West Nile (WNV), Dengue, and Zika virus infections after treatment with oral antibiotics (Abx) that depleted the gut microbiota. Abx treatment impaired the development of optimal T cell responses, with decreased levels of WNV-specific CD8 + T cells associated with increased infection and immunopathology. Abx treatments that resulted in enhanced WNV susceptibility generated changes in the overall structure of the gut bacterial community and in the abundance of specific bacterial taxa. As little as 3 days of treatment with ampicillin was sufficient to alter host immunity and WNV outcome. Our results identify oral Abx therapy as a potential environmental determinant of systemic viral disease, and they raise the possibility that perturbation of the gut microbiota may have deleterious consequences for subsequent flavivirus infections.
In Brief
Thackray et al. observed increased susceptibility to West Nile, Zika, and Dengue virus infections following oral antibiotic treatment in mice. Antibiotics altered the bacterial abundance and community structure and the development of optimal T cell immunity. These data suggest that antibiotics may have deleterious consequences for subsequent flavivirus infections.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity.
- Record: found
- Abstract: found
- Article: not found
Microbiota regulates immune defense against respiratory tract influenza A virus infection.
- Record: found
- Abstract: found
- Article: not found