- Record: found
- Abstract: found
- Article: found
Catamenial Epilepsy: Discovery of an Extrasynaptic Molecular Mechanism for Targeted Therapy
Read this article at
Abstract
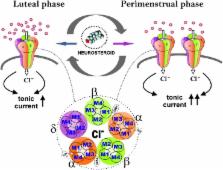
Catamenial epilepsy is a type of refractory epilepsy characterized by seizure clusters around perimenstrual or periovulatory period. The pathophysiology of catamenial epilepsy still remains unclear, yet there are few animal models to study this gender-specific disorder. The pathophysiology of perimenstrual catamenial epilepsy involves the withdrawal of the progesterone-derived GABAergic neurosteroids due to the decline in progesterone level at the time of menstruation. These manifestations can be faithfully reproduced in rodents by specific neuroendocrine manipulations. Since mice and rats, like humans, have ovarian cycles with circulating hormones, they appear to be suitable animal models for studies of perimenstrual seizures. Recently, we created specific experimental models to mimic perimenstrual seizures. Studies in rat and mouse models of catamenial epilepsy show enhanced susceptibility to seizures or increased seizure exacerbations following neurosteroid withdrawal. During such a seizure exacerbation period, there is a striking decrease in the anticonvulsant effect of commonly prescribed antiepileptics, such as benzodiazepines, but an increase in the anticonvulsant potency of exogenous neurosteroids. We discovered an extrasynaptic molecular mechanism of catamenial epilepsy. In essence, extrasynaptic δGABA-A receptors are upregulated during perimenstrual-like neuroendocrine milieu. Consequently, there is enhanced antiseizure efficacy of neurosteroids in catamenial models because δGABA-A receptors confer neurosteroid sensitivity and greater seizure protection. Molecular mechanisms such as these offer a strong rationale for the clinical development of a neurosteroid replacement therapy for catamenial epilepsy.
Related collections
Most cited references79
- Record: found
- Abstract: found
- Article: not found
Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety.
- Record: found
- Abstract: found
- Article: not found
Neurosteroids: endogenous role in the human brain and therapeutic potentials.
- Record: found
- Abstract: found
- Article: not found