- Record: found
- Abstract: found
- Article: found
Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans
Read this article at
Significance
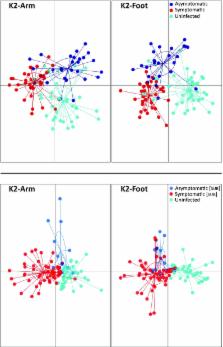
Malaria elimination efforts are hindered by the prevalence of asymptomatic infections, which frequently go undetected and untreated. Consequently, there is a pressing need for improved diagnostic screening methods. Based on extensive collections of skin odors from human populations in Kenya, we report broad and consistent effects of malaria infection on human volatile emissions. Furthermore, we found that predictive models based on machine learning algorithms reliably determined infection status based on volatile biomarkers and, critically, identified asymptomatic infections with 100% sensitivity, even in the case of low-level infections not detectable by microscopy. These findings suggest that volatile biomarkers have significant potential for the development of robust, noninvasive screening methods for detecting symptomatic and asymptomatic malaria infections under field conditions.
Abstract
Malaria remains among the world’s deadliest diseases, and control efforts depend critically on the availability of effective diagnostic tools, particularly for the identification of asymptomatic infections, which play a key role in disease persistence and may account for most instances of transmission but often evade detection by current screening methods. Research on humans and in animal models has shown that infection by malaria parasites elicits changes in host odors that influence vector attraction, suggesting that such changes might yield robust biomarkers of infection status. Here we present findings based on extensive collections of skin volatiles from human populations with high rates of malaria infection in Kenya. We report broad and consistent effects of malaria infection on human volatile profiles, as well as significant divergence in the effects of symptomatic and asymptomatic infections. Furthermore, predictive models based on machine learning algorithms reliably determined infection status based on volatile biomarkers. Critically, our models identified asymptomatic infections with 100% sensitivity, even in the case of low-level infections not detectable by microscopy, far exceeding the performance of currently available rapid diagnostic tests in this regard. We also identified a set of individual compounds that emerged as consistently important predictors of infection status. These findings suggest that volatile biomarkers may have significant potential for the development of a robust, noninvasive screening method for detecting malaria infections under field conditions.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
The silent threat: asymptomatic parasitemia and malaria transmission.
- Record: found
- Abstract: found
- Article: not found
Odourant reception in the malaria mosquito Anopheles gambiae
- Record: found
- Abstract: found
- Article: not found