- Record: found
- Abstract: found
- Article: found
N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia : A randomized controlled trial
Read this article at
Abstract
Oxidative stress is considered to be part of the pathogenic mechanism for community-acquired pneumonia (CAP) and is closely linked to inflammation. Attenuation of oxidative stress would be expected to reduce pulmonary damage. Antioxidants have been found to be effective in alleviating lung injury and protecting against damage of other organs.
The aim of the study was to compare the effect of adding N-acetylcysteine (NAC) to conventional treatment versus conventional treatment on oxidative stress, inflammatory factors, and radiological changes in CAP patients.
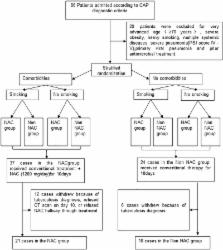
Eligible CAP patients at Weihai Municipal Hospital were stratified and randomly assigned to either NAC group or non-NAC group between August 2016 and March 2017. The NAC group received conventional treatment for pneumonia and NAC (1200 mg/d). Thenon-NAC group received conventional therapy. malondialdehyde (MDA), superoxide dismutase (SOD), total antioxidant capacity (TAOC), tumor necrosis factor-α (TNF-α), and computed tomography (CT) images were evaluated at baseline and after treatment. The primary endpoint indicators were the changes in oxidative stress parameters (MDA, TAOC, SOD) and TNF-α after treatment in the NAC group compared with those in the non-NAC group. The secondary endpoint indicator was any difference in CT scores after treatment in the NAC group compared with the non-NAC group.
Baseline levels of MDA, TAOC, SOD, and TNF-α were similar between the 2 groups before treatment. Plasma levels of MDA and TNF-α decreased more ( P < .05 MDA:p 0.004, TNF-α:p <0.001) in the NAC group than the non-NAC group, and there was a reliable increase in TAOC content (p 0.005). There was no significant difference in increased plasma SOD activity between the groups (p 0.368), and the NAC group did not show a greater improvement from CT scores. No NAC-related adverse effects were observed.
Addition of NAC therapy for CAP patients reduced MDA and TNF-α and increased TAOC. Treatment with NAC may help to reduce oxidative and inflammatory damage in pneumonia patients.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Oxidative stress and regulation of glutathione in lung inflammation.
- Record: found
- Abstract: found
- Article: not found