- Record: found
- Abstract: found
- Article: found
Structure and compatibility of a magnesium electrolyte with a sulphur cathode
Read this article at
Abstract
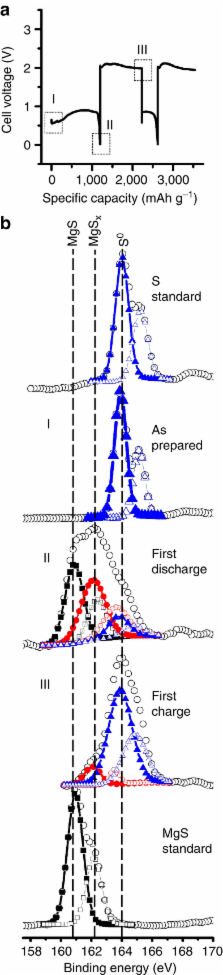
Magnesium metal is an ideal rechargeable battery anode material because of its high volumetric energy density, high negative reduction potential and natural abundance. Coupling Mg with high capacity, low-cost cathode materials such as electrophilic sulphur is only possible with a non-nucleophilic electrolyte. Here we show how the crystallization of the electrochemically active species formed from the reaction between hexamethyldisilazide magnesium chloride and aluminum trichloride enables the synthesis of a non-nucleophilic electrolyte. Furthermore, crystallization was essential in the identification of the electroactive species, [Mg 2(μ-Cl) 3·6THF] +, and vital to improvements in the voltage stability and coulombic efficiency of the electrolyte. X-ray photoelectron spectroscopy analysis of the sulphur electrode confirmed that the electrochemical conversion between sulphur and magnesium sulfide can be successfully performed using this electrolyte.
Abstract
 Magnesium is an ideal rechargeable battery anode material, but coupling it with a
low-cost sulphur cathode, requires a non-nucleophilic electrolyte. Kim
et al. prepare a non-nucleophilic electrolyte from hexamethyldisilazide magnesium chloride
and aluminium trichloride, and show its compatibility with a sulphur cathode.
Magnesium is an ideal rechargeable battery anode material, but coupling it with a
low-cost sulphur cathode, requires a non-nucleophilic electrolyte. Kim
et al. prepare a non-nucleophilic electrolyte from hexamethyldisilazide magnesium chloride
and aluminium trichloride, and show its compatibility with a sulphur cathode.
Related collections
Most cited references6
- Record: found
- Abstract: not found
- Article: not found
Nonaqueous liquid electrolytes for lithium-based rechargeable batteries.
- Record: found
- Abstract: found
- Article: not found
Prototype systems for rechargeable magnesium batteries.
- Record: found
- Abstract: found
- Article: not found