- Record: found
- Abstract: found
- Article: found
A hybrid semiconducting organosilica-based O 2 nanoeconomizer for on-demand synergistic photothermally boosted radiotherapy
Read this article at
Abstract
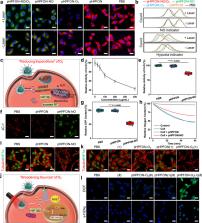
The outcome of radiotherapy is significantly restricted by tumor hypoxia. To overcome this obstacle, one prevalent solution is to increase intratumoral oxygen supply. However, its effectiveness is often limited by the high metabolic demand for O 2 by cancer cells. Herein, we develop a hybrid semiconducting organosilica-based O 2 nanoeconomizer pHPFON-NO/O 2 to combat tumor hypoxia. Our solution is twofold: first, the pHPFON-NO/O 2 interacts with the acidic tumor microenvironment to release NO for endogenous O 2 conservation; second, it releases O 2 in response to mild photothermal effect to enable exogenous O 2 infusion. Additionally, the photothermal effect can be increased to eradicate tumor residues with radioresistant properties due to other factors. This “reducing expenditure of O 2 and broadening sources” strategy significantly alleviates tumor hypoxia in multiple ways, greatly enhances the efficacy of radiotherapy both in vitro and in vivo, and demonstrates the synergy between on-demand temperature-controlled photothermal and oxygen-elevated radiotherapy for complete tumor response.
Abstract
Tumor hypoxia is a major limitation in radiotherapy, and strategies to address this often fail due to high oxygen consumption. Here, the authors report a nanomaterial assembly for the simultaneous reduction in mitochondrial respiration and to supply oxygen to potentiate radiotherapy.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Otto Warburg's contributions to current concepts of cancer metabolism.
- Record: found
- Abstract: found
- Article: not found
The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence.
- Record: found
- Abstract: found
- Article: not found