- Record: found
- Abstract: found
- Article: found
Cardiac Atrial Circadian Rhythms in PERIOD2::LUCIFERASE and per1:luc Mice: Amplitude and Phase Responses to Glucocorticoid Signaling and Medium Treatment
Read this article at
Abstract
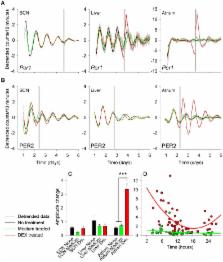
Circadian rhythms in cardiac function are apparent in e.g., blood pressure, heart rate, and acute adverse cardiac events. A circadian clock in heart tissue has been identified, but entrainment pathways of this clock are still unclear. We cultured tissues of mice carrying bioluminescence reporters of the core clock genes, period 1 or 2 ( per1 luc or PER2 LUC) and compared in vitro responses of atrium to treatment with medium and a synthetic glucocorticoid (dexamethasone [DEX]) to that of the suprachiasmatic nucleus (SCN) and liver. We observed that PER2 LUC, but not per1 luc is rhythmic in atrial tissue, while both per1 luc and PER2 LUC exhibit rhythmicity in other cultured tissues. In contrast to the SCN and liver, both per1 luc and PER2 LUC bioluminescence amplitudes were increased in response to DEX treatment, and the PER2 LUC amplitude response was dependent on the time of treatment. Large phase-shift responses to both medium and DEX treatments were observed in the atrium, and phase responses to medium treatment were not attributed to serum content but the treatment procedure itself. The phase-response curves of atrium to both DEX and medium treatments were found to be different to the liver. Moreover, the time of day of the culturing procedure itself influenced the phase of the circadian clock in each of the cultured tissues, but the magnitude of this response was uniquely large in atrial tissue. The current data describe novel entrainment signals for the atrial circadian clock and specifically highlight entrainment by mechanical treatment, an intriguing observation considering the mechanical nature of cardiac tissue.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Resetting of circadian time in peripheral tissues by glucocorticoid signaling.
- Record: found
- Abstract: found
- Article: not found
Extensive and divergent circadian gene expression in liver and heart.
- Record: found
- Abstract: found
- Article: not found