- Record: found
- Abstract: found
- Article: found
Titin‐based mechanosensing modulates muscle hypertrophy
Read this article at
Abstract
Background
Titin is an elastic sarcomeric filament that has been proposed to play a key role in mechanosensing and trophicity of muscle. However, evidence for this proposal is scarce due to the lack of appropriate experimental models to directly test the role of titin in mechanosensing.
Methods
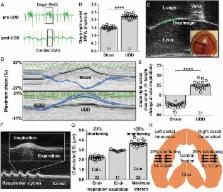
We used unilateral diaphragm denervation (UDD) in mice, an in vivo model in which the denervated hemidiaphragm is passively stretched by the contralateral, innervated hemidiaphragm and hypertrophy rapidly occurs.
Results
In wildtype mice, the denervated hemidiaphragm mass increased 48 ± 3% after 6 days of UDD, due to the addition of both sarcomeres in series and in parallel. To test whether titin stiffness modulates the hypertrophy response, RBM20 ΔRRM and Ttn ΔIAjxn mouse models were used, with decreased and increased titin stiffness, respectively. RBM20 ΔRRM mice (reduced stiffness) showed a 20 ± 6% attenuated hypertrophy response, whereas the Ttn ΔIAjxn mice (increased stiffness) showed an 18 ± 8% exaggerated response after UDD. Thus, muscle hypertrophy scales with titin stiffness. Protein expression analysis revealed that titin‐binding proteins implicated previously in muscle trophicity were induced during UDD, MARP1 & 2, FHL1, and MuRF1.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: found
Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2017
- Record: found
- Abstract: found
- Article: not found
Calcium-dependent molecular spring elements in the giant protein titin.
- Record: found
- Abstract: found
- Article: not found