- Record: found
- Abstract: found
- Article: found
Alzheimer's disease: the amyloid hypothesis and the Inverse Warburg effect
Read this article at
Abstract
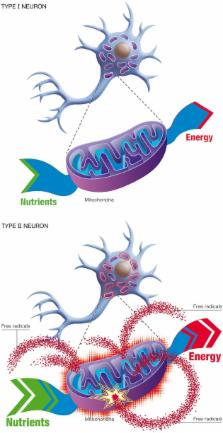
Epidemiological and biochemical studies show that the sporadic forms of Alzheimer's disease (AD) are characterized by the following hallmarks: (a) An exponential increase with age; (b) Selective neuronal vulnerability; (c) Inverse cancer comorbidity. The present article appeals to these hallmarks to evaluate and contrast two competing models of AD: the amyloid hypothesis (a neuron-centric mechanism) and the Inverse Warburg hypothesis (a neuron-astrocytic mechanism). We show that these three hallmarks of AD conflict with the amyloid hypothesis, but are consistent with the Inverse Warburg hypothesis, a bioenergetic model which postulates that AD is the result of a cascade of three events—mitochondrial dysregulation, metabolic reprogramming (the Inverse Warburg effect), and natural selection. We also provide an explanation for the failures of the clinical trials based on amyloid immunization, and we propose a new class of therapeutic strategies consistent with the neuroenergetic selection model.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization.
- Record: found
- Abstract: found
- Article: not found
Ageing and neuronal vulnerability.
- Record: found
- Abstract: found
- Article: not found