- Record: found
- Abstract: found
- Article: not found
p38 MAPK-dependent shaping of the keratin cytoskeleton in cultured cells
Read this article at
Abstract
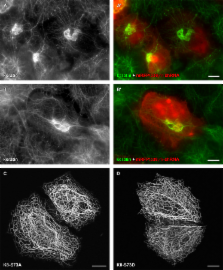
Plasticity of the resilient keratin intermediate filament cytoskeleton is an important prerequisite for epithelial tissue homeostasis. Here, the contribution of stress-activated p38 MAPK to keratin network organization was examined in cultured cells. It was observed that phosphorylated p38 colocalized with keratin granules that were rapidly formed in response to orthovanadate. The same p38 p recruitment was noted during mitosis, in various stress situations and in cells producing mutant keratins. In all these situations keratin 8 became phosphorylated on S73, a well-known p38 target site. To demonstrate that p38-dependent keratin phosphorylation determines keratin organization, p38 activity was pharmacologically and genetically modulated: up-regulation induced keratin granule formation, whereas down-regulation prevented keratin filament network disassembly. Furthermore, transient p38 inhibition also inhibited keratin filament precursor formation and mutant keratin granule dissolution. Collectively, the rapid and reversible effects of p38 activity on keratin phosphorylation and organization in diverse physiological, stress, and pathological situations identify p38-dependent signalling as a major intermediate filament–regulating pathway.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: not found
Specificity and mechanism of action of some commonly used protein kinase inhibitors.
- Record: found
- Abstract: found
- Article: not found
New consensus nomenclature for mammalian keratins
- Record: found
- Abstract: found
- Article: not found
