- Record: found
- Abstract: found
- Article: not found
Overcoming Ovarian Cancer Drug Resistance with a Cold Responsive Nanomaterial

Read this article at
Abstract
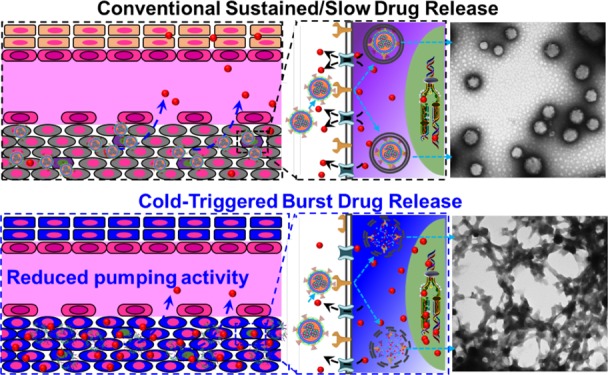
Drug resistance due to overexpression of membrane transporters in cancer cells and the existence of cancer stem cells (CSCs) is a major hurdle to effective and safe cancer chemotherapy. Nanoparticles have been explored to overcome cancer drug resistance. However, drug slowly released from nanoparticles can still be efficiently pumped out of drug-resistant cells. Here, a hybrid nanoparticle of phospholipid and polymers is developed to achieve cold-triggered burst release of encapsulated drug. With ice cooling to below ∼12 °C for both burst drug release and reduced membrane transporter activity, binding of the drug with its target in drug-resistant cells is evident, while it is minimal in the cells kept at 37 °C. Moreover, targeted drug delivery with the cold-responsive nanoparticles in combination with ice cooling not only can effectively kill drug-resistant ovarian cancer cells and their CSCs in vitro but also destroy both subcutaneous and orthotopic ovarian tumors in vivo with no evident systemic toxicity.
Abstract
A cold-responsive hybrid nanoparticle of phospholipid and polymers is developed to overcome the multidrug resistance of cancer stem cells with ice cooling.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Drug resistance and the solid tumor microenvironment.

- Record: found
- Abstract: found
- Article: found
Tumor-selective catalytic nanomedicine by nanocatalyst delivery

- Record: found
- Abstract: found
- Article: found
