- Record: found
- Abstract: found
- Article: found
Delirium epidemiology in critical care (DECCA): an international study
Read this article at
Abstract
Introduction
Delirium is a frequent source of morbidity in intensive care units (ICUs). Most data on its epidemiology is from single-center studies. Our aim was to conduct a multicenter study to evaluate the epidemiology of delirium in the ICU.
Methods
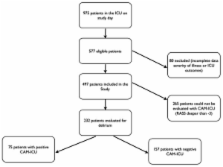
A 1-day point-prevalence study was undertaken in 104 ICUs from 11 countries in South and North America and Spain.
Results
In total, 975 patients were screened, and 497 fulfilled inclusion criteria and were enrolled (median age, 62 years; 52.5% men; 16.7% and 19.9% for ICU and hospital mortality); 64% were admitted to the ICU because of medical causes, and sepsis was the main diagnosis ( n = 76; 15.3%). In total, 265 patients were sedated with the Richmond agitation and sedation scale (RASS) deeper than -3, and only 232 (46.6%) patients could be evaluated with the confusion-assessment method for the ICU. The prevalence of delirium was 32.3%. Compared with patients without delirium, those with the diagnosis of delirium had a greater severity of illness at admission, demonstrated by higher sequential organ-failure assessment (SOFA ( P = 0.004)) and simplified acute physiology score 3 (SAPS3) scores ( P < 0.0001). Delirium was associated with increased ICU (20% versus 5.7%; P = 0.002) and hospital mortality (24 versus 8.3%; P = 0.0017), and longer ICU ( P < 0.0001) and hospital length of stay (LOS) (22 (11 to 40) versus 7 (4 to 18) days; P < 0.0001). Previous use of midazolam ( P = 0.009) was more frequent in patients with delirium. On multivariate analysis, delirium was independently associated with increased ICU mortality (OR = 3.14 (1.26 to 7.86); CI, 95%) and hospital mortality (OR = 2.5 (1.1 to 5.7); CI, 95%).
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Incidence, risk factors and consequences of ICU delirium.
- Record: found
- Abstract: found
- Article: not found
SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description
- Record: found
- Abstract: found
- Article: not found