- Record: found
- Abstract: found
- Article: found
KRIT1 Loss-Of-Function Associated with Cerebral Cavernous Malformation Disease Leads to Enhanced S-Glutathionylation of Distinct Structural and Regulatory Proteins
Read this article at
Abstract
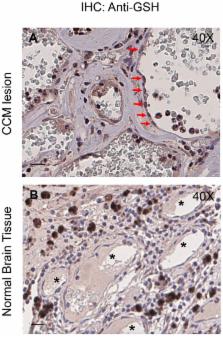
Loss-of-function mutations in the KRIT1 gene are associated with the pathogenesis of cerebral cavernous malformations (CCMs), a major cerebrovascular disease still awaiting therapies. Accumulating evidence demonstrates that KRIT1 plays an important role in major redox-sensitive mechanisms, including transcriptional pathways and autophagy, which play major roles in cellular homeostasis and defense against oxidative stress, raising the possibility that KRIT1 loss has pleiotropic effects on multiple redox-sensitive systems. Using previously established cellular models, we found that KRIT1 loss-of-function affects the glutathione (GSH) redox system, causing a significant decrease in total GSH levels and increase in oxidized glutathione disulfide (GSSG), with a consequent deficit in the GSH/GSSG redox ratio and GSH-mediated antioxidant capacity. Redox proteomic analyses showed that these effects are associated with increased S-glutathionylation of distinct proteins involved in adaptive responses to oxidative stress, including redox-sensitive chaperonins, metabolic enzymes, and cytoskeletal proteins, suggesting a novel molecular signature of KRIT1 loss-of-function. Besides providing further insights into the emerging pleiotropic functions of KRIT1, these findings point definitively to KRIT1 as a major player in redox biology, shedding new light on the mechanistic relationship between KRIT1 loss-of-function and enhanced cell sensitivity to oxidative stress, which may eventually lead to cellular dysfunctions and CCM disease pathogenesis.
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: not found
Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes.
- Record: found
- Abstract: found
- Article: not found
Glutathione dysregulation and the etiology and progression of human diseases.
- Record: found
- Abstract: found
- Article: not found