- Record: found
- Abstract: found
- Article: not found
Stress-dependent relocalization of translationally primed mRNPs to cytoplasmic granules that are kinetically and spatially distinct from P-bodies
Read this article at
Abstract
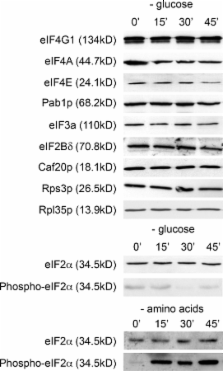
Cytoplasmic RNA granules serve key functions in the control of messenger RNA (mRNA) fate in eukaryotic cells. For instance, in yeast, severe stress induces mRNA relocalization to sites of degradation or storage called processing bodies (P-bodies). In this study, we show that the translation repression associated with glucose starvation causes the key translational mediators of mRNA recognition, eIF4E, eIF4G, and Pab1p, to resediment away from ribosomal fractions. These mediators then accumulate in P-bodies and in previously unrecognized cytoplasmic bodies, which we define as EGP-bodies. Our kinetic studies highlight the fundamental difference between EGP- and P-bodies and reflect the complex dynamics surrounding reconfiguration of the mRNA pool under stress conditions. An absence of key mRNA decay factors from EGP-bodies points toward an mRNA storage function for these bodies. Overall, this study highlights new potential control points in both the regulation of mRNA fate and the global control of translation initiation.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
P bodies and the control of mRNA translation and degradation.
- Record: found
- Abstract: found
- Article: not found
