- Record: found
- Abstract: found
- Article: found
Effect of amniotic fluid stem cell transplantation on the recovery of bladder dysfunction in spinal cord-injured rats
Abstract
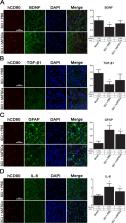
The effects of human amniotic fluid stem cell (hAFSC) transplantation on bladder function and molecular changes in spinal cord-injured (SCI) rats were investigated. Four groups were studied: sham and SCI plus phosphate-buffered saline (SCI + PBS), human embryonic kidney 293 (HEK293) cells, and hAFSCs transplantation. In SCI + PBS rat bladders, cystometry showed increased peak voiding pressure, voiding volume, bladder capacity, residual volume, and number of non-voiding contractions, and the total elastin/collagen amount was increased but collagen concentration was decreased at days 7 and 28. Immunoreactivity and mRNA levels of IGF-1, TGF-β1, and β3-adrenoceptor were increased at days 7 and/or 28. M2 immunoreactivity and M3 mRNA levels of muscarinic receptor were increased at day 7. M2 immunoreactivity was increased, but M2/M3 mRNA and M3 immunoreactivity levels were decreased at day 28. Brain derived-neurotrophic factor mRNA was increased, but immunoreactivity was decreased at day 7. HEK293 cell transplantation caused no difference compared to SCI + PBS group. hAFSCs co-localized with neural cell markers and expressed BDNF, TGF-β1, GFAP, and IL-6. The present results showed that SCI bladders released IGF-1 and TGF-β1 to stimulate elastin and collagen for bladder wall remodelling, and hAFSC transplantation improved these changes, which involved the mechanisms of BDNF, muscarinic receptors, and β3-adrenoceptor expression.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Targeting recovery: priorities of the spinal cord-injured population.
- Record: found
- Abstract: found
- Article: not found
Isolation of amniotic stem cell lines with potential for therapy.
- Record: found
- Abstract: found
- Article: not found