- Record: found
- Abstract: found
- Article: found
Responding to policy makers’ evaluation needs: combining experimental and quasi-experimental approaches to estimate the impact of performance based financing in Burkina Faso
Read this article at
Abstract
Background
The last two decades have seen a growing recognition of the need to expand the impact evaluation toolbox from an exclusive focus on randomized controlled trials to including quasi-experimental approaches. This appears to be particularly relevant when evaluation complex health interventions embedded in real-life settings often characterized by multiple research interests, limited researcher control, concurrently implemented policies and interventions, and other internal validity-threatening circumstances. To date, however, most studies described in the literature have employed either an exclusive experimental or an exclusive quasi-experimental approach.
Methods
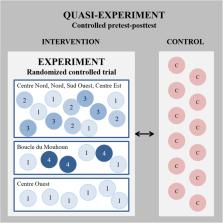
This paper presents the case of a study design exploiting the respective advantages of both approaches by combining experimental and quasi-experimental elements to evaluate the impact of a Performance-Based Financing (PBF) intervention in Burkina Faso. Specifically, the study employed a quasi-experimental design (pretest-posttest with comparison) with a nested experimental component (randomized controlled trial). A difference-in-differences approach was used as the main analytical strategy.
Discussion
We aim to illustrate a way to reconcile scientific and pragmatic concerns to generate policy-relevant evidence on the intervention’s impact, which is methodologically rigorous in its identification strategy but also considerate of the context within which the intervention took place. In particular, we highlight how we formulated our research questions, ultimately leading our design choices, on the basis of the knowledge needs expressed by the policy and implementing stakeholders. We discuss methodological weaknesses of the design arising from contextual constraints and the accommodation of various interests, and how we worked ex-post to address them to the best extent possible to ensure maximal accuracy and credibility of our findings. We hope that our case may be inspirational for other researchers wishing to undertake research in settings where field circumstances do not appear to be ideal for an impact evaluation.
Related collections
Most cited references26
- Record: found
- Abstract: not found
- Article: not found
Bootstrap-Based Improvements for Inference with Clustered Errors
- Record: found
- Abstract: not found
- Article: not found
Nonparametric standard errors and confidence intervals
- Record: found
- Abstract: found
- Article: not found