- Record: found
- Abstract: found
- Article: found
Developmental Increase of Neocortical Presynaptic Efficacy via Maturation of Vesicle Replenishment
Read this article at
Abstract
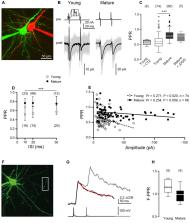
The efficacy of neocortical synapses to transmit during bursts of action potentials (APs) increases during development but the underlying mechanisms are largely unclear. We investigated synaptic efficacy at synapses between layer 5 pyramidal neurons (L5PNs) during development, using paired recordings, presynaptic two-photon Ca 2+ imaging, and numerical simulations. Our data confirm a developmental increase in paired-pulse ratios (PPRs). Independent of age, Ca 2+ imaging revealed no AP invasion failures and linear summation of presynaptic Ca 2+ transients, making differences in Ca 2+ signaling an unlikely reason for developmental changes in PPR. Cumulative excitatory postsynaptic current (EPSC) amplitudes indicate that neither the size of the readily-releasable pool (RRP) nor replenishment rates were different between age groups, while the time-courses of depression differed significantly. At young synapses, EPSCs depressed rapidly to near steady-state during the first four APs, and synaptic failures (F syn) increased from 0 to 30%. At mature synapses this drop was significantly slower and strongly biphasic, such that near steady-state depression was reached not before 18 APs with F syn remaining between 0 and 5%. While young synapses reliably transmitted during pairs of APs, albeit with strong depression, mature synapses maintained near 100% transfer efficacy with significantly less depression during high-frequency bursts of APs. Our analysis indicates that at mature synapses a replenishment pool (RepP) is responsible for their high efficacy during bursting activity, while this RepP is functionally immature at young synapses. Hence, our data provide evidence that the functional maturation of a RepP underlies increasing synaptic efficacy during the development of an excitatory cortical synapse.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Short-term synaptic plasticity.
- Record: found
- Abstract: found
- Article: not found
Synaptic vesicle pools.
- Record: found
- Abstract: found
- Article: not found