- Record: found
- Abstract: found
- Article: not found
Nephrocalcinosis is a risk factor for kidney failure in primary hyperoxaluria
Read this article at
Abstract
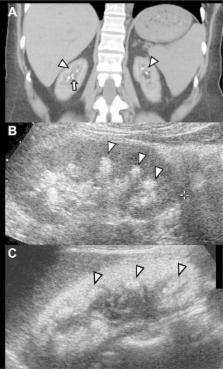
Stone formation and nephrocalcinosis are both very common features of primary hyperoxaluria, yet the extent of each disease varies markedly between patients. Here we studied whether kidney damage from nephrocalcinosis and/or stone related events contributed to end stage kidney disease (ESKD). Clinical information was analyzed from 348 patients enrolled in the Rare Kidney Stone Consortium Primary Hyperoxaluria registry and included demographic, laboratory and imaging features. Among all patients there were 277 with type 1, 37 with type 2, and 34 with type 3 primary hyperoxaluria. Overall, 58% passed a stone (mean 0.3/year) and one or more urologic procedures were required by 70% of patients (mean 0.15/year). Nephrocalcinosis was found in 34% of patients, including 41% with type 1 primary hyperoxaluria. High urine oxalate was associated with increased risk for both nephrocalcinosis and stone number, while low urine citrate was a risk factor for stone events and stone number. After adjustment for the type of primary hyperoxaluria, diagnosis by family screening and age at first image, the overall adjusted hazard ratio for ESKD among those with a history of nephrocalcinosis was 1.7 [95% CI 1.0–3.0], while the risk was 4.0 [1.9–8.5] for new onset nephrocalcinosis during follow-up. In contrast, the number of stones and stone events were not significantly associated with ESKD risk. Thus, nephrolithiasis and nephrocalcinosis appear to be pathophysiologically distinct entities. The presence of nephrocalcinosis implies increased risk for ESKD.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Hereditary causes of kidney stones and chronic kidney disease.
- Record: found
- Abstract: found
- Article: not found
Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys.
- Record: found
- Abstract: found
- Article: not found
