- Record: found
- Abstract: found
- Article: found
The Unfolded Protein Response: A Key Player in Zika Virus-Associated Congenital Microcephaly
Read this article at
Abstract
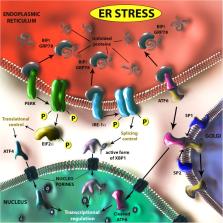
Zika virus (ZIKV) is a mosquito-borne virus that belongs to the Flaviviridae family, together with dengue, yellow fever, and West Nile viruses. In the wake of its emergence in the French Polynesia and in the Americas, ZIKV has been shown to cause congenital microcephaly. It is the first arbovirus which has been proven to be teratogenic and sexually transmissible. Confronted with this major public health challenge, the scientific and medical communities teamed up to precisely characterize the clinical features of congenital ZIKV syndrome and its underlying pathophysiological mechanisms. This review focuses on the critical impact of the unfolded protein response (UPR) on ZIKV-associated congenital microcephaly. ZIKV infection of cortical neuron progenitors leads to high endoplasmic reticulum (ER) stress. This results in both the stalling of indirect neurogenesis, and UPR-dependent neuronal apoptotic death, and leads to cortical microcephaly. In line with these results, the administration of molecules inhibiting UPR prevents ZIKV-induced cortical microcephaly. The discovery of the link between ZIKV infection and UPR activation has a broader relevance, since this pathway plays a crucial role in many distinct cellular processes and its induction by ZIKV may account for several reported ZIKV-associated defects.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
Endoplasmic reticulum stress: cell life and death decisions.
- Record: found
- Abstract: not found
- Article: not found
An update on Zika virus infection
- Record: found
- Abstract: found
- Article: not found