- Record: found
- Abstract: found
- Article: found
Activation of autophagy by FOXO3 regulates redox homeostasis during osteogenic differentiation
Read this article at
ABSTRACT
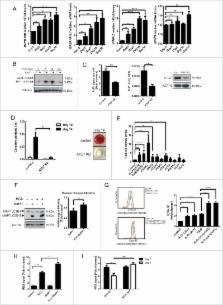
Bone remodeling is a continuous physiological process that requires constant generation of new osteoblasts from mesenchymal stem cells (MSCs). Differentiation of MSCs to osteoblast requires a metabolic switch from glycolysis to increased mitochondrial respiration to ensure the sufficient energy supply to complete this process. As a consequence of this increased mitochondrial metabolism, the levels of endogenous reactive oxygen species (ROS) rise. In the current study we analyzed the role of forkhead box O3 (FOXO3) in the control of ROS levels in human MSCs (hMSCs) during osteogenic differentiation. Treatment of hMSCs with H 2O 2 induced FOXO3 phosphorylation at Ser294 and nuclear translocation. This ROS-mediated activation of FOXO3 was dependent on mitogen-activated protein kinase 8 (MAPK8/JNK) activity. Upon FOXO3 downregulation, osteoblastic differentiation was impaired and hMSCs lost their ability to control elevated ROS levels. Our results also demonstrate that in response to elevated ROS levels, FOXO3 induces autophagy in hMSCs. In line with this, impairment of autophagy by autophagy-related 7 (ATG7) knockdown resulted in a reduced capacity of hMSCs to regulate elevated ROS levels, together with a reduced osteoblast differentiation. Taken together our findings are consistent with a model where in hMSCs, FOXO3 is required to induce autophagy and thereby reduce elevated ROS levels resulting from the increased mitochondrial respiration during osteoblast differentiation. These new molecular insights provide an important contribution to our better understanding of bone physiology.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis.
- Record: found
- Abstract: found
- Article: not found
Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes.
- Record: found
- Abstract: found
- Article: not found