- Record: found
- Abstract: found
- Article: found
Novel heterozygous C243Y A20/TNFAIP3 gene mutation is responsible for chronic inflammation in autosomal-dominant Behçet's disease
Read this article at
Abstract
Objective
Although Behçet's disease (BD) is a chronic inflammatory disorder of uncertain aetiology, the existence of familial BD with autosomal-dominant traits suggests that a responsibility gene (or genes) exists. We investigated a Japanese family with a history of BD to search for pathogenic mutations underlying the biological mechanisms of BD.
Methods
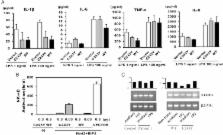
6 patients over 4 generations who had suffered from frequent oral ulcers, genital ulcers and erythaema nodosum-like lesions in the skin were assessed. Whole-exome sequencing was performed on genomic DNA, and cytokine production was determined from stimulated mononuclear cells. Inflammatory cytokine secretion and Nod2-mediated NF-κB activation were analysed using the transfected cells.
Results
By whole-exome sequencing, we identified a common heterozygous missense mutation in A20/TNFAIP3, a gene known to regulate NF-κB signalling, for which all affected family members carried a heterozygous C243Y mutation in the ovarian tumour domain. Mononuclear cells obtained from the proband and his mother produced large amounts of interleukin 1β, IL-6 and tumour necrosis factor α (TNF-a) on stimulation as compared with those from normal controls. Although inflammatory cytokine secretion was suppressed by wild-type transfected cells, it was suppressed to a much lesser extent by mutated C243Y A20/TNFAIP3-transfected cells. In addition, impaired suppression of Nod2-mediated NF-κB activation by C243Y A20/TNFAIP3 was observed.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis
- Record: found
- Abstract: found
- Article: not found