- Record: found
- Abstract: found
- Article: found
Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals
Read this article at
Abstract
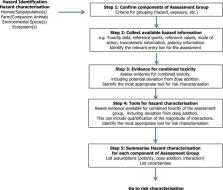
This Guidance document describes harmonised risk assessment methodologies for combined exposure to multiple chemicals for all relevant areas within EFSA's remit, i.e. human health, animal health and ecological areas. First, a short review of the key terms, scientific basis for combined exposure risk assessment and approaches to assessing (eco)toxicology is given, including existing frameworks for these risk assessments. This background was evaluated, resulting in a harmonised framework for risk assessment of combined exposure to multiple chemicals. The framework is based on the risk assessment steps (problem formulation, exposure assessment, hazard identification and characterisation, and risk characterisation including uncertainty analysis), with tiered and stepwise approaches for both whole mixture approaches and component‐based approaches. Specific considerations are given to component‐based approaches including the grouping of chemicals into common assessment groups, the use of dose addition as a default assumption, approaches to integrate evidence of interactions and the refinement of assessment groups. Case studies are annexed in this guidance document to explore the feasibility and spectrum of applications of the proposed methods and approaches for human and animal health and ecological risk assessment. The Scientific Committee considers that this Guidance is fit for purpose for risk assessments of combined exposure to multiple chemicals and should be applied in all relevant areas of EFSA's work. Future work and research are recommended.
Abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1589/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1602/full
Related collections
Most cited references133
- Record: found
- Abstract: found
- Article: not found
Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment.
- Record: found
- Abstract: found
- Article: not found
The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds.

- Record: found
- Abstract: found
- Article: found