- Record: found
- Abstract: found
- Article: found
Epidemiology and Pathophysiology of Glomerular C4d Staining in Native Kidney Biopsies

Read this article at
Abstract
Introduction
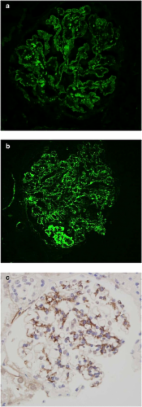
Routine C4d staining in renal transplantation has stimulated its use in kidney biopsies with glomerulonephritis (GN). Methodical description on staining patterns in the native kidney is not available.
Methods
We retrospectively evaluated C4d staining in formalin-fixed paraffin-embedded sections from 519 native kidney biopsies (bx) with and without glomerular disease.
Results
Strong C4d staining was consistently present in immune-complex GN, including lupus nephritis (LN) ( n = 68), membranous GN ( n = 24), membranoproliferative glomerulonephritis (MPGN) pattern ( n = 22), fibrillary GN ( n = 3), and proliferative GN with monoclonal IgG ( n = 3). C4d stained all cases of postinfectious GN ( n = 7) amyloidosis ( n = 20) and C1q GN ( n = 3). In contrast, IgA nephropathy (IgAN) ( n = 34), was negative in 62% of bx, with the rest staining variably. The E1 Oxford classification score correlated with capillary wall C4d staining ( P = 0.05). C4d marked the glomerular and arteriolar lesions in thrombotic microangiopathy (TMA; n = 16), the glomerular sclerotic segments in focal segmental glomerulosclerosis (FSGS; n = 77), and marked areas of necrosis in crescentic GN ( n = 21). In diabetic glomerulopathy ( n = 70), C4d marked advanced insudative lesions but was negative otherwise. C4d weakly stained the mesangium, or was negative in normal biopsies ( n = 13), minimal change disease (MCD; n = 21), thin basement membrane disease ( n = 20), Alport ( n = 3), IgM nephropathy ( n = 2), C3 glomerulopathy ( n = 5), acute interstitial nephritis ( n = 12), acute tubular necrosis ( n = 22), ischemic glomerulopathy/nephrosclerosis ( n = 23), and other miscellaneous processes ( n = 14). Staining in tubular basement membranes and peritubular capillaries was most common in lupus.
Graphical abstract
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
The role of complement in inflammatory diseases from behind the scenes into the spotlight.