- Record: found
- Abstract: found
- Article: found
Potential impact of the implementation of multiple-criteria decision analysis (MCDA) on the Polish pricing and reimbursement process of orphan drugs
Read this article at
Abstract
Background
The objective of this study was to assess the potential impact of the implementation of multiple-criteria decision analysis (MCDA) on the Polish pricing and reimbursement (P&R) process with regard to orphan drugs.
Methods
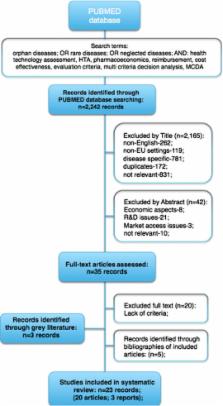
A four step approach was designed. Firstly, a systematic literature review was conducted to select the MCDA criteria. Secondly, a database of orphan drugs was established. Thirdly, health technology appraisals (HTA recommendations) were categorized and an MCDA appraisal was conducted. Finally, a comparison of HTA and MCDA outcomes was carried out. An MCDA outcome was considered positive if more than 50 % of the maximum number of points was reached (base case). In the sensitivity analysis, 25 % and 75 % thresholds were tested as well.
Results
Out of 2242 publications, 23 full-text articles were included. The final MCDA tool consisted of ten criteria. In total, 27 distinctive drug-indication pairs regarding 21 drugs were used for the study. Six negative and 21 positive HTA recommendations were issued. In the base case, there were 19 positive MCDA outcomes. Of the 27 cases, there were 12 disagreements between the HTA and MCDA outcomes, the majority of which related to positive HTA guidance for negative MCDA outcomes. All drug-indication pairs with negative HTA recommendations were appraised positively in the MCDA framework. Economic details were available for 12 cases, of which there were 9 positive MCDA outcomes. Amongst the 12 drug-indication pairs, two were negatively appraised in the HTA process, with positive MCDA guidance, and two were appraised in the opposite direction.
Conclusions
An MCDA approach may lead to different P&R outcomes compared to a standard HTA process. On the one hand, enrichment of the list of decision making criteria means further scrutiny of a given health technology and as such increases the odds of a negative P&R outcome. On the other hand, it may uncover additional values and as such increase the odds of positive P&R outcomes.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Cost-effectiveness analysis and the consistency of decision making: evidence from pharmaceutical reimbursement in australia (1991 to 1996).
- Record: found
- Abstract: found
- Article: not found
Multi-criteria clinical decision support: A primer on the use of multiple criteria decision making methods to promote evidence-based, patient-centered healthcare.
- Record: found
- Abstract: found
- Article: not found