- Record: found
- Abstract: found
- Article: found
Crystalline deposition in the cornea and conjunctiva secondary to long-term clofazimine therapy in a leprosy patient
letter
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Dear Editor,
The continuous introduction of new systemic medications and dosing changes in current
drug regimens has resulted in ever-increasing reports of ocular toxicities.[1] We
report an unusual side-effect of long term therapy with clofazimine, which caused
numerous polychromatic crystalline deposits within the cornea and conjunctiva in a
leprosy patient.
A 30-year-old woman, case of lepromatous leprosy with recurrent type II lepra reaction,
on tablet clofazimine 100 mg/ day was referred to us from dermatology clinic for brownish
discoloration of conjunctiva. She was diagnosed as a case of lepromatous leprosy three
years ago and started on multi drug therapy-multi bacillary (MDT-MB), which included
clofazimine 50 mg/day and 300mg/ month as pulse dose. She was not on any other medication.
After three months of treatment, she developed type II lepra reaction and was treated
with clofazimine and corticosteroids. The dose of clofazimine was 300 mg/day for two
months, which was tapered over the next three months. Over the next two years, she
developed two more episodes of type II lepra reaction for which she had again received
reactional doses of clofazimine. Estimated cumulative dose of clofazimine was 891.0
gm.
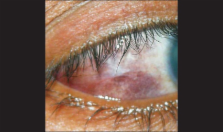
Her best corrected visual acuity was 20/50 in both eyes. On slit lamp examination,
brownish-red discoloration of peripheral cornea and conjunctiva in inter-palpebral
region was noted [Fig. 1]. There were multiple polychromatic crystalline deposits
scattered diffusely over peripheral cornea and conjunctiva of both eyes [Figs. 2 and
3]. The lens had Grade 2 nuclear sclerosis in both eyes but no similar deposits. Fundoscopy
was normal in both eyes. She also had reddish-brown discoloration of skin. Clofazimine
therapy was stopped after two months as treatment of type II lepra reaction was completed.
On follow-up after 6 months of discontinuing clofazimine, best corrected visual activity
was 20/50 in both eyes and the conjunctival and corneal crystalline deposits had decreased
along with conjunctival discoloration [Fig. 4]. The absence of any other known cause
of crystalline corneal deposits confirmed long-term clofazimine therapy as a cause
of crystalline deposition in the cornea and conjunctiva.
Figure 1
Brownish-red discoloration of peripheral cornea and conjunctiva
Figure 2
Polychromatic crystalline deposits over conjunctiva () represents crystalline deposits
Figure 3
Polychromatic crystalline deposits over cornea. () represents crystalline deposits
Figure 4
Follow-up at 6 months
Corneal stromal deposition may develop from a number of medications such as clofazimine,
gold, immunoglobulins, indomethacin, phenothiazines, retinoids, sparfloxacin, and
silver. The deposits of drugs and drug metabolites within corneal stroma may be predominantly
pigmented, crystalline, or refractile.[1] Crystalline deposits in cornea are reported
following exogenous immunoglobulin therapy and the crystals appear in mid periphery
in annular fashion.[2] Gokhale observed multiple refractile crystalline deposits in
the corneal stroma following prolonged topical sparfloxacin therapy.[3]
Corneal and conjunctival changes have been reported previously in association with
clofazimine therapy. Kaur et al., observed conjunctival pigmentation in 46% and corneal
pigmentation in 53% patients treated with clofazimine for 6–24 months.[4] Our patient
had polychromatic crystalline deposits along with brownish-red discoloration in bulbar
conjunctiva and peripheral cornea, which did not affect the vision. Careful literature
search revealed that only one such case is reported by Font et al.,[5] having estimated
cumulative dose of clofazimine of 219 gm as compared to 891 gm in our patient In the
case reported by Font et al., ultrastructural study of conjunctival biopsy demonstrated
that many of fibroblasts and macrophages contained rectangular or rhomboidal empty
spaces corresponding to crystals, which ranged from 1.5 to 7 μm in length.[5]
In greater than 1% of patients on clofazimine therapy diminished vision and ocular
dryness, burning, itching, and irritation have been reported, which were absent in
our case. Craythorn et al.,[6] reported macular pigmentary abnormalities but in our
patient macula was normal.
Further studies of clofazimine-treated patients are necessary in order to determine
the frequency and spectrum of corneal and conjunctival abnormalities associated with
the drug. It is suggested that patients being treated with clofazimine should undergo
periodic ophthalmic examination. Clofazimine-induced crystalline keratopathy should
be included in the differential diagnosis of crystalline deposits of cornea and conjunctiva.
Related collections
Most cited references6
- Record: found
- Abstract: found
- Article: not found
Drug-induced corneal complications.
Anthony Aldave, Allan Hollander (2004)
- Record: found
- Abstract: found
- Article: not found
Polychromatic corneal and conjunctival crystals secondary to clofazimine therapy in a leper.
Ramon L Font, Warren Sobol, Alice Matoba (1989)
- Record: found
- Abstract: found
- Article: not found
Clofazimine-induced bull's-eye retinopathy.
J Craythorn, M. Swartz, D J Creel (2016)