- Record: found
- Abstract: found
- Article: found
Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development
Read this article at
Abstract
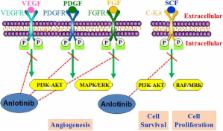
Anlotinib is a new, orally administered tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptors (PDGFR), and c-kit. Compared to the effect of placebo, it improved both progression-free survival (PFS) and overall survival (OS) in a phase III trial in patients with advanced non-small-cell lung cancer (NSCLC), despite progression of the cancer after two lines of prior treatments. Recently, the China Food and Drug Administration (CFDA) approved single agent anlotinib as a third-line treatment for patients with advanced NSCLC. Moreover, a randomized phase IIB trial demonstrated that anlotinib significantly prolonged the median PFS in patients with advanced soft tissue sarcoma (STS). Anlotinib also showed promising efficacy in patients with advanced medullary thyroid carcinoma and metastatic renal cell carcinoma (mRCC). The tolerability profile of anlotinib is similar to that of other tyrosine kinase inhibitors that target VEGFR and other tyrosine kinase-mediated pathways; however, anlotinib has a significantly lower incidence of grade 3 or higher side effects compared to that of sunitinib. We review the rationale, clinical evidence, and future perspectives of anlotinib for the treatment of multiple cancers.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial.
- Record: found
- Abstract: found
- Article: not found
Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial
- Record: found
- Abstract: found
- Article: not found