- Record: found
- Abstract: found
- Article: found
Effect of peritoneal dialysis fluid containing osmo-metabolic agents on human endothelial cells
Abstract
Background
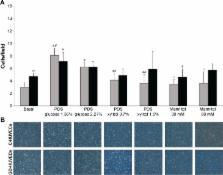
The use of glucose as the only osmotic agent in peritoneal dialysis (PD) solutions (PDSs) is believed to exert local (peritoneal) and systemic detrimental actions, particularly in diabetic PD patients. To improve peritoneal biocompatibility, we have developed more biocompatible PDSs containing xylitol and carnitine along with significantly less amounts of glucose and have tested them in cultured Human Vein Endothelial Cells (HUVECs) obtained from the umbilical cords of healthy (C) and gestational diabetic (GD) mothers.
Methods
Primary C- and GD-HUVECs were treated for 72 hours with our PDSs (xylitol 0.7% and 1.5%, whereas carnitine and glucose were fixed at 0.02% and 0.5%, respectively) and two glucose-based PDSs (glucose 1.36% or 2.27%). We examined their effects on endothelial cell proliferation (cell count), viability (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay), intracellular nitro-oxidative stress (peroxynitrite levels), Vascular Cell Adhesion Molecule-1 and Intercellular Adhesion Molecule-1 membrane exposure (flow cytometry), and HUVEC-monocyte interactions (U937 adhesion assay).
Results
Compared to glucose-based PDSs, our in vitro studies demonstrated that the tested PDSs did not change the proliferative potential both in C- and GD-HUVECs. Moreover, our PDSs significantly improved endothelial cell viability, compared to glucose-based PDSs and basal condition. Notably, glucose-based PDSs significantly increased the intracellular peroxynitrite levels, Vascular Cell Adhesion Molecule-1 and Intercellular Adhesion Molecule-1 membrane exposure, and endothelial cell–monocyte interactions in both C- and GD-HUVECs, as compared with our experimental PDSs.
Conclusion
Present results show that in control and diabetic human endothelial cell models, xylitol–carnitine-based PDSs do not cause cytotoxicity, nitro-oxidative stress, and inflammation as caused by hypertonic glucose-based PDSs. Since xylitol and carnitine are also known to favorably affect glucose homeostasis, these findings suggest that our PDSs may represent a desirable hypertonic solution even for diabetic patients in PD.
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study.
- Record: found
- Abstract: found
- Article: not found
Predictors of loss of residual renal function among new dialysis patients.
- Record: found
- Abstract: found
- Article: not found