- Record: found
- Abstract: found
- Article: found
E3 ubiquitin ligase siah-1 nuclear accumulation is critical for homocysteine-induced impairment of C6 astroglioma cells
Read this article at
Abstract
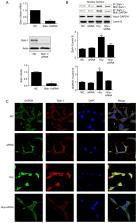
Elevated plasma homocysteine (Hcy), known as hyperhomocysteinemia (HHcy), is an independent risk factor for neurodegenerative diseases. Hcy, even at a low concentration, can promote free radical formation and increase oxidative stress, leading to neuronal death, which may be an important mechanism underlying the pathogenesis of neurodegenerative diseases. Although several reports have indicated that the nuclear translocation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) may be involved in Hcy-induced apoptosis, the exact mechanism remains to be fully elucidated. The siah E3 ubiquitin protein ligase 1 (siah-1) gene was found to be critical for the translocation of GAPDH from the cytoplasm to the nucleus. In the present study, the role of siah-1 was investigated in the nuclear translocation of GAPDH in rat C6 astroglioma cells treated with Hcy. C6 cells were treated with various concentrations of Hcy for 48 h and the expression level of siah-1 was examined using reverse transcription-quantitative polymerase chain reaction and western blotting analysis. In addition, the subcellular localization of siah-1 and GAPDH and the interaction between these two factors were investigated by immunofluorescence staining and co-immunoprecipitation assay, respectively. The results showed that Hcy at a high concentration increased the expression of siah-1 and induced nuclear translocation of siah-1 and GAPDH. In addition, siah-1 knockdown by siah-1 small interfering RNA significantly decreased the Hcy-induced nuclear accumulation of GAPDH and inhibited the impairment of C6 cells. These findings suggest that siah-1 is involved in Hcy-induced cell damage by promoting the nuclear translocation of GAPDH.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity.
- Record: found
- Abstract: found
- Article: not found
Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease.
- Record: found
- Abstract: found
- Article: not found