- Record: found
- Abstract: found
- Article: found
RNAi-mediated knockdown of the voltage gated sodium ion channel TcNa v causes mortality in Tribolium castaneum
Read this article at
Abstract
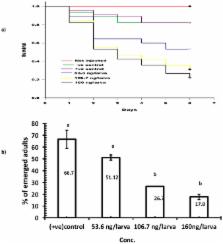
The voltage-gated sodium ion channel (VGSC) belongs to the largest superfamily of ion channels. Since VGSCs play key roles in physiological processes they are major targets for effective insecticides. RNA interference (RNAi) is widely used to analyse gene function, but recently, it has shown potential to contribute to novel strategies for selectively controlling agricultural insect pests. The current study evaluates the delivery of dsRNA targeted to the sodium ion channel paralytic A (TcNa v) gene in Tribolium castaneum as a viable means of controlling this insect pest. Delivery of TcNa v dsRNA caused severe developmental arrest with larval mortalities up to 73% post injection of dsRNA. Injected larvae showed significant (p < 0.05) knockdown in gene expression between 30–60%. Expression was also significantly (p < 0.05) reduced in pupae following injection causing 30% and 42% knockdown for early and late pupal stages, respectively. Oral delivery of dsRNA caused dose-dependant mortalities of between 19 and 51.34%; this was accompanied by significant (p < 0.05) knockdown in gene expression following 3 days of continuous feeding. The majority of larvae injected with, or fed, dsRNA died during the final larval stage prior to pupation. This work provides evidence of a viable RNAi-based strategy for insect control.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Control of coleopteran insect pests through RNA interference.
- Record: found
- Abstract: found
- Article: not found
Ingested double-stranded RNAs can act as species-specific insecticides.
- Record: found
- Abstract: found
- Article: not found