- Record: found
- Abstract: found
- Article: found
Stability, safety, and transcorneal mechanistic studies of ophthalmic lyophilized cyclosporine-loaded polymeric micelles
Abstract
Introduction
Cyclosporine-A (CsA) is generally used as an immunosuppressant and is also prescribed for some ophthalmic applications such as vernal keratoconjunctivitis and dry eye. However, it is limited clinically due to its low aqueous solubility and ocular bioavailability.
Methods
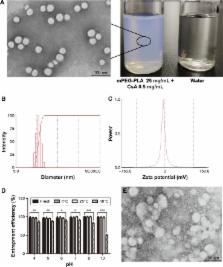
In this work, lyophilized methoxy poly(ethylene glycol)-poly(lactide) (mPEG-PLA) polymer micelles were prepared for ophthalmic formulations as a promising nanocarrier for hydrophobic drugs like CsA. A mPEG-PLA diblock polymer was synthesized by ring opening polymerization and CsA was loaded into mPEG-PLA micelles by a simple film dispersion method. A uniform design of experiments was utilized to optimize the final formulation. The obtained formulation was characterized for diameter (57.0±3.2 nm), entrapment efficiency % (98.51±1.4), and in vitro release. Moreover, incorporating the stabilizer mPEG2000 could increase the in vitro stability of the lyophilized CsA-loaded mPEG-PLA micelles.
Results
Results showed a sustained release of CsA from the micelles. Drug concentration and time-dependent cytotoxicity of human corneal epithelial-2 cells was observed. Additionally, the transcorneal mechanism of mPEG-PLA micelles was studied and the results showed that the mPEG-PLA micelles mainly absorbed by a paracellular pathway via corneal epithelial cells.
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC).
- Record: found
- Abstract: found
- Article: not found
Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation.
- Record: found
- Abstract: found
- Article: not found