- Record: found
- Abstract: found
- Article: found
Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone
Read this article at
Abstract
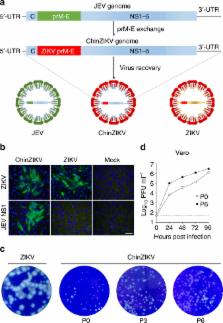
The global spread of Zika virus (ZIKV) and its unexpected association with congenital defects necessitates the rapid development of a safe and effective vaccine. Here we report the development and characterization of a recombinant chimeric ZIKV vaccine candidate (termed ChinZIKV) that expresses the prM-E proteins of ZIKV using the licensed Japanese encephalitis live-attenuated vaccine SA14-14-2 as the genetic backbone. ChinZIKV retains its replication activity and genetic stability in vitro, while exhibiting an attenuation phenotype in multiple animal models. Remarkably, immunization of mice and rhesus macaques with a single dose of ChinZIKV elicits robust and long-lasting immune responses, and confers complete protection against ZIKV challenge. Significantly, female mice immunized with ChinZIKV are protected against placental and fetal damage upon ZIKV challenge during pregnancy. Overall, our study provides an alternative vaccine platform in response to the ZIKV emergency, and the safety, immunogenicity, and protection profiles of ChinZIKV warrant further clinical development.
Abstract
Given the recent Zika virus (ZIKV) epidemic, development of an effective vaccine is of high importance. Here, the authors use a licensed live-attenuated flavivirus vaccine backbone to develop a ZIKV vaccine and determine immunogenicity, safety and protection profiles in different animal models.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys.
- Record: found
- Abstract: found
- Article: not found
Rapid development of a DNA vaccine for Zika virus.
- Record: found
- Abstract: found
- Article: not found