- Record: found
- Abstract: found
- Article: found
Insulin and Glucagon Regulate Pancreatic α-Cell Proliferation
Read this article at
Abstract
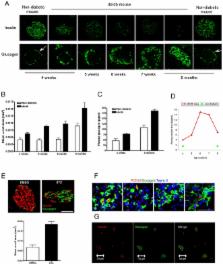
Type 2 diabetes mellitus (T2DM) results from insulin resistance and β-cell dysfunction, in the setting of hyperglucagonemia. Glucagon is a 29 amino acid peptide hormone, which is secreted from pancreatic α cells: excessively high circulating levels of glucagon lead to excessive hepatic glucose output. We investigated if α-cell numbers increase in T2DM and what factor (s) regulate α-cell turnover. Lepr db/Lepr db (db/db) mice were used as a T2DM model and αTC1 cells were used to study potential α-cell trophic factors. Here, we demonstrate that in db/db mice α-cell number and plasma glucagon levels increased as diabetes progressed. Insulin treatment (EC50 = 2 nM) of α cells significantly increased α-cell proliferation in a concentration-dependent manner compared to non-insulin-treated α cells. Insulin up-regulated α-cell proliferation through the IR/IRS2/AKT/mTOR signaling pathway, and increased insulin-mediated proliferation was prevented by pretreatment with rapamycin, a specific mTOR inhibitor. GcgR antagonism resulted in reduced rates of cell proliferation in αTC1 cells. In addition, blockade of GcgRs in db/db mice improved glucose homeostasis, lessened α-cell proliferation, and increased intra-islet insulin content in β cells in db/db mice. These studies illustrate that pancreatic α-cell proliferation increases as diabetes develops, resulting in elevated plasma glucagon levels, and both insulin and glucagon are trophic factors to α-cells. Our current findings suggest that new therapeutic strategies for the treatment of T2DM may include targeting α cells and glucagon.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
SREBP Activity Is Regulated by mTORC1 and Contributes to Akt-Dependent Cell Growth
- Record: found
- Abstract: found
- Article: not found
Selective versus total insulin resistance: a pathogenic paradox.
- Record: found
- Abstract: found
- Article: not found