- Record: found
- Abstract: found
- Article: found
Cabergoline, Dopamine D2 Receptor Agonist, Prevents Neuronal Cell Death under Oxidative Stress via Reducing Excitotoxicity
Read this article at
Abstract
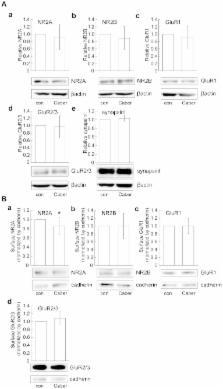
Several lines of evidence demonstrate that oxidative stress is involved in the pathogenesis of neurodegenerative diseases, including Parkinson's disease. Potent antioxidants may therefore be effective in the treatment of such diseases. Cabergoline, a dopamine D2 receptor agonist and antiparkinson drug, has been studied using several cell types including mesencephalic neurons, and is recognized as a potent radical scavenger. Here, we examined whether cabergoline exerts neuroprotective effects against oxidative stress through a receptor-mediated mechanism in cultured cortical neurons. We found that neuronal death induced by H 2O 2 exposure was inhibited by pretreatment with cabergoline, while this protective effect was eliminated in the presence of a dopamine D 2 receptor inhibitor, spiperone. Activation of ERK1/2 by H 2O 2 was suppressed by cabergoline, and an ERK signaling pathway inhibitor, U0126, similarly protected cortical neurons from cell death. This suggested the ERK signaling pathway has a critical role in cabergoline-mediated neuroprotection. Furthermore, increased extracellular levels of glutamate induced by H 2O 2, which might contribute to ERK activation, were reduced by cabergoline, while inhibitors for NMDA receptor or L-type Ca 2+ channel demonstrated a survival effect against H 2O 2. Interestingly, we found that cabergoline increased expression levels of glutamate transporters such as EAAC1. Taken together, these results suggest that cabergoline has a protective effect on cortical neurons via a receptor-mediated mechanism including repression of ERK1/2 activation and extracellular glutamate accumulation induced by H 2O 2.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Localization of neuronal and glial glutamate transporters.
- Record: found
- Abstract: found
- Article: not found
Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways.
- Record: found
- Abstract: found
- Article: not found