- Record: found
- Abstract: found
- Article: found
Tailored-Dose Baclofen in the Management of Alcoholism: A Retrospective Study of 144 Outpatients Followed for 3 Years in a French General Practice
Read this article at
Abstract
Background: More information is needed about the efficacy and safety of long-term baclofen in the treatment of alcohol use disorders. The objective of this study was to assess the effect of treatment with tailored-dose baclofen on alcohol consumption in patients with alcohol use disorders followed for 3 years after first initiating baclofen treatment.
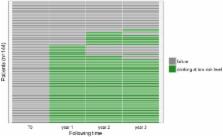
Methods: This retrospective descriptive cohort included outpatients followed in a French general practice clinic for 3 years and treated with tailored-dose baclofen to reduce or eliminate alcohol consumption. At 3 years, treatment was considered successful if alcohol consumption was at or below levels defined as low-risk by the WHO (≤ 40 g/d in men and ≤ 20 g/d in women).
Results: The study population included 144 patients (88 men and 56 women). The participants' mean age was 46 ± 11 years and mean daily alcohol intake before treatment was 167 ± 77 grams. At the end of the study, treatment was successful for 91 (63.2%) patients. Participants' mean dose of baclofen at the end of study period was 100 ± 101 mg/d. We identified 75 (52.1%) patients for whom treatment was successful at each annual follow-up appointment: at 1, 2, and 3 years. The mean maximum dose of baclofen over follow-up of the 144 patients was 211 ± 99 mg/d (dose range: 40 mg/d to 520 mg/d).
Conclusion: In this study, tailored-dose baclofen appears to be an effective treatment in patients with alcohol use disorders, with sustainable effect over time (3 years). There are many adverse effects but they are consistent with those already described in the literature.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study.
- Record: found
- Abstract: found
- Article: not found
A Randomized, Placebo-Controlled Study of High-Dose Baclofen in Alcohol-Dependent Patients-The ALPADIR Study.

- Record: found
- Abstract: found
- Article: found