- Record: found
- Abstract: found
- Article: found
A review on hepatitis D: From virology to new therapies
Read this article at
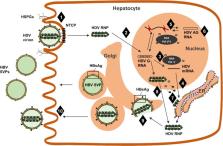
Graphical abstract
Highlights
-
•
Hepatitis D virus is a defective virus, dependent on hepatitis B virus for its assembly.
-
•
Hepatitis D virus infection affects 62–72 million people worldwide.
-
•
Chronic hepatitis D is the most severe chronic viral hepatitis.
-
•
Current interferon-based antiviral treatments have dismal efficiency and are poorly tolerated.
-
•
Host-targeting molecules inhibiting the viral life cycle are currently in clinical development.
Abstract
Hepatitis delta virus (HDV) is a defective virus that requires the hepatitis B virus (HBV) to complete its life cycle in human hepatocytes. HDV virions contain an envelope incorporating HBV surface antigen protein and a ribonucleoprotein containing the viral circular single-stranded RNA genome associated with both forms of hepatitis delta antigen, the only viral encoded protein. Replication is mediated by the host cell DNA-dependent RNA polymerases. HDV infects up to72 million people worldwide and is associated with an increased risk of severe and rapidly progressive liver disease. Pegylated interferon-alpha is still the only available treatment for chronic hepatitis D, with poor tolerance and dismal success rate. Although the development of antivirals inhibiting the viral replication is challenging, as HDV does not possess its own polymerase, several antiviral molecules targeting other steps of the viral life cycle are currently under clinical development: Myrcludex B, which blocks HDV entry into hepatocytes, lonafarnib, a prenylation inhibitor that prevents virion assembly, and finally REP 2139, which is thought to inhibit HBsAg release from hepatocytes and interact with hepatitis delta antigen. This review updates the epidemiology, virology and management of HDV infection.
Related collections
Most cited references151

- Record: found
- Abstract: found
- Article: found
Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus
- Record: found
- Abstract: found
- Article: not found
Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study
- Record: found
- Abstract: found
- Article: not found
