- Record: found
- Abstract: found
- Article: found
Targeting Antigens to Dec-205 on Dendritic Cells Induces Immune Protection in Experimental Colitis in Mice
Abstract
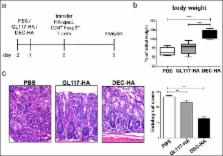
The endocytotic c-type lectin receptor DEC-205 is highly expressed on immature dendritic cells. In previous studies, it was shown that antigen-targeting to DEC-205 is a useful tool for the induction of antigen-specific Foxp3 + regulatory T cells and thereby can prevent inflammatory processes. However, whether this approach is sufficient to mediate tolerance in mucosal tissues like the gut is unknown. In this study, we established a new mouse model in which the adoptive transfer of naive hemagglutinin (HA)-specific CD4 +Foxp3 – T cells into VILLIN-HA transgenic mice leads to severe colitis. To analyze if antigen-targeting to DEC-205 could protect against inflammation of the gut, VILLIN-HA transgenic mice were injected with an antibody–antigen complex consisting of the immunogenic HA 110–120 peptide coupled to an α-DEC-205 antibody (DEC-HA) before adoptive T cell transfer. DEC-HA-treated mice showed significantly less signs of intestinal inflammation as was demonstrated by reduced loss of body weight and histopathology in the gut. Strikingly, abrogated intestinal inflammation was mediated via the conversion of naive HA-specific CD4 +Foxp3 – T cells into HA-specific CD4 +Foxp3 + regulatory T cells. In this study, we provide evidence that antigen-targeting to DEC-205 can be utilized for the induction of tolerance in mucosal organs that are confronted with large numbers of exogenous antigens.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Regulatory T cell lineage specification by the forkhead transcription factor foxp3.
- Record: found
- Abstract: found
- Article: not found
Dendritic Cells Induce Peripheral T Cell Unresponsiveness under Steady State Conditions in Vivo
- Record: found
- Abstract: found
- Article: not found