- Record: found
- Abstract: found
- Article: not found
Dynamic Behavior of the Active and Inactive States of the Adenosine A 2A Receptor
Read this article at
Abstract

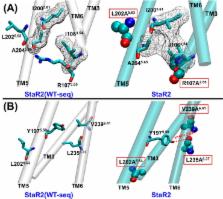
The adenosine A 2A receptor (A 2AR) belongs to the superfamily of membrane proteins called the G-protein-coupled receptors (GPCRs) that form one of the largest superfamilies of drug targets. Deriving thermostable mutants has been one of the strategies used for crystallization of A 2AR in both the agonist and antagonist bound conformational states. The crystal structures do not reveal differences in the activation mechanism of the mutant receptors compared to the wild type receptor, that have been observed experimentally. These differences stem from the dynamic behavior of the mutant receptors. Furthermore, it is not understood how the mutations confer thermostability. Since these details are difficult to obtain from experiments, we have used atomic level simulations to elucidate the dynamic behavior of the agonist and antagonist bound mutants as well the wild type A 2AR. We found that significant enthalpic contribution leads to stabilization of both the inactive state (StaR2) and active-like state (GL31) thermostable mutants of A 2AR. Stabilization resulting from mutations of bulky residues to alanine is due to the formation of interhelical hydrogen bonds and van der Waals packing that improves the transmembrane domain packing. The thermostable mutant GL31 shows less movement of the transmembrane helix TM6 with respect to TM3 than the wild type receptor. While restricted dynamics of GL31 is advantageous in its purification and crystallization, it could also be the reason why these mutants are not efficient in activating the G proteins. We observed that the calculated stress on each residue is higher in the wild type receptor compared to the thermostable mutants, and this stress is required for activation to occur. Thus, reduced dynamic behavior of the thermostable mutants leading to lowered activation of these receptors originates from reduced stress on each residue. Finally, accurate calculation of the change in free energy for single mutations shows good correlation with the change in the measured thermostability. These results provide insights into the effect of mutations that can be incorporated in deriving thermostable mutants for other GPCRs.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor.
- Record: found
- Abstract: found
- Article: not found
International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update.
- Record: found
- Abstract: found
- Article: not found
