- Record: found
- Abstract: found
- Article: found
Contributions of epithelial-mesenchymal transition and cancer stem cells to the development of castration resistance of prostate cancer
Read this article at
Abstract
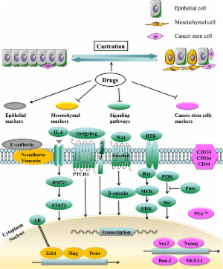
An important clinical challenge in prostate cancer therapy is the inevitable transition from androgen-sensitive to castration-resistant and metastatic prostate cancer. Albeit the androgen receptor (AR) signaling axis has been targeted, the biological mechanism underlying the lethal event of androgen independence remains unclear. New emerging evidences indicate that epithelial-to-mesenchymal transition (EMT) and cancer stem cells (CSCs) play crucial roles during the development of castration-resistance and metastasis of prostate cancer. Notably, EMT may be a dynamic process. Castration can induce EMT that may enhance the stemness of CSCs, which in turn results in castration-resistance and metastasis. Reverse of EMT may attenuate the stemness of CSCs and inhibit castration-resistance and metastasis. These prospective approaches suggest that therapies target EMT and CSCs may cast a new light on the treatment of castration-resistant prostate cancer (CRPC) in the future. Here we review recent progress of EMT and CSCs in CRPC.
Related collections
Most cited references80
- Record: found
- Abstract: found
- Article: not found
Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity.
- Record: found
- Abstract: found
- Article: not found
Hedgehog signalling in prostate regeneration, neoplasia and metastasis.
- Record: found
- Abstract: found
- Article: not found