- Record: found
- Abstract: found
- Article: found
The Immunometabolomic Interface Receptor Hydroxycarboxylic Acid Receptor 2 Mediates the Therapeutic Effects of Dimethyl Fumarate in Autoantibody-Induced Skin Inflammation
Read this article at
Abstract
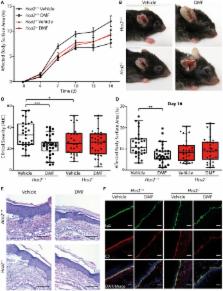
The drug dimethyl fumarate (DMF) is in clinical use for the treatment of psoriasis and multiple sclerosis. In addition, it has recently been demonstrated to ameliorate skin pathology in mouse models of pemphigoid diseases, a group of autoimmune blistering diseases of the skin and mucous membranes. However, the mode of action of DMF in inflammatory skin diseases has remained elusive. Therefore, we have investigated here the mechanisms by which DMF improves skin pathology, using the antibody transfer model of bullous pemphigoid-like epidermolysis bullosa acquisita (EBA). Experimental EBA was induced by transfer of antibodies against collagen VII that triggered the infiltration of immune cells into the skin and led to inflammatory skin lesions. DMF treatment reduced the infiltration of neutrophils and monocytes into the skin explaining the improved disease outcome in DMF-treated animals. Upon ingestion, DMF is converted to monomethyl fumarate that activates the hydroxycarboxylic acid receptor 2 (HCA 2). Interestingly, neutrophils and monocytes expressed Hca2. To investigate whether the therapeutic effect of DMF in EBA is mediated by HCA 2, we administered oral DMF to Hca2-deficient mice ( Hca2 −/−) and wild-type littermates ( Hca2 +/+) and induced EBA. DMF treatment ameliorated skin lesions in Hca2 +/+ but not in Hca2 −/− animals. These findings demonstrate that HCA 2 is a molecular target of DMF treatment in EBA and suggest that HCA 2 activation limits skin pathology by inhibiting the infiltration of neutrophils and monocytes into the skin.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages.
- Record: found
- Abstract: found
- Article: not found
PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect.
- Record: found
- Abstract: found
- Article: not found