- Record: found
- Abstract: found
- Article: found
The design of novel inhibitors for treating cancer by targeting CDC25B through disruption of CDC25B-CDK2/Cyclin A interaction using computational approaches
Read this article at
Abstract
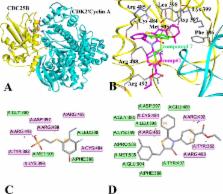
Cell division cycle 25B is a key cell cycle regulator and widely considered as potent clinical drug target for cancers. This research focused on identifying potential compounds in theory which are able to disrupt transient interactions between CDC25B and its CDK2/Cyclin A substrate.
By using the method of ZDOCK and RDOCK, the most optimized 3D structure of CDK2/Cyclin A in complex with CDC25B was constructed and validated using two methods: 1) the superimposition of proteins; 2) analysis of the hydrogen bond distances of Arg 488(N1)-Asp 206(OD1), Arg 492(NE)-Asp 206(OD1), Arg 492(N1)-Asp 206(OD2) and Tyr 497(NE)-Asp 210(OD1). A series of new compounds was gained through searching the fragment database derived from ZINC based on the known inhibitor-compound 7 by the means of “replace fragment” technique. The compounds acquired via meeting the requirements of the absorption, distribution, metabolism, and excretion (ADME) predictions. Finally, 12 compounds with better binding affinity were identified. The comp#1, as a representative, was selected to be synthesized and assayed for their CDC25B inhibitory activities. The comp#1 exhibited mild inhibitory activities against human CDC25B with IC 50 values at about 39.02 μM. Molecular Dynamic (MD) simulation revealed that the new inhibitor-comp#1 had favorable conformations for binding to CDC25B and disturbing the interactions between CDC25B and CDK2/Cyclin A.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
ZDOCK: an initial-stage protein-docking algorithm.
- Record: found
- Abstract: found
- Article: not found
Prediction of drug absorption using multivariate statistics.
- Record: found
- Abstract: found
- Article: not found