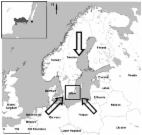
Recent outbreaks of West Nile virus infection or avian influenza indicate that birds participate in the ecology of zoonotic infections, an important cause of illness and death in humans and animals ( 1 ). The emergence of these threats underscores the need for understanding the maintenance of bird-associated infections in nature, which is prerequisite for disease prevention. Migratory birds are known to carry several microbial agents of human disease, including viruses, chlamydiae, and enterobacteria ( 2 , 3 ). Evidence of the last 2 decades indicates that birds in North America and Eurasia host vectorborne pathogens, such as Anaplasma species and Lyme borreliosis (LB) spirochetes ( 4 – 6 ). LB is the most common vectorborne zoonosis in temperate regions of the Northern Hemisphere and is transmitted to humans by Ixodes ticks ( 7 ). Borrelia spirochetes infect naive Ixodes larvae when they feed on a reservoir host and are transmitted back to the reservoir population by infected nymphs. Rodent species, such as the white-footed mouse (Peromyscus leucopus) in the northeastern United States and Apodemus and Clethrionomys species in continental Europe, are common hosts of both immature ticks and LB spirochetes ( 8 , 9 ). However, recent field vaccination and biodiversity studies suggest that alternative hosts play a greater role than expected in the natural cycle of LB ( 10 , 11 ). In comparison with studies of mammals as LB reservoirs, few studies have been conducted on the role of birds as hosts of Borrelia. The natural cycle of LB spirochetes, in particular Borrelia garinii, involves seabirds in northern Europe and game birds in the United Kingdom, which are the most studied models ( 12 , 13 ). However, the relationship between migratory passerine birds and Borrelia is less understood. Although experimental studies on avian infection have been conducted ( 14 – 17 ), less is known about reservoir competence of natural bird populations, especially those that could transmit ticks that frequently bite humans ( 5 , 18 – 20 ). Information that would allow comparison of the reservoir importance of bird and other vertebrate populations is not available or is controversial. Although 1 modeling study found that the frequency of LB cases was positively correlated with species diversity of ground-dwelling birds ( 21 ), other studies have found the contribution of birds in hosting and infecting ticks to be low ( 22 , 23 ). Another uncertainty is epidemiologic implications of LB group spirochetes associated with birds. For example, birds in Europe are reservoirs of B. valaisiana, which has not been associated with disease ( 19 ). In the present study, we characterized tick infestation and Borrelia transmission from migratory passerine birds captured in southern Sweden to further define their importance as reservoirs and disseminators of these spirochetes. We found that these birds are hosts of epidemiologically important vector ticks and Borrelia species. However, exposure of birds to ticks, which depends on feeding habits, determines their effectiveness as Borrelia reservoirs. Materials and Methods Bird Capture and Tick Collection Birds were captured at Ottenby Bird Observatory (http://www.sofnet.org/ofstn/Engelska) at the southern point of Öland Island in the Baltic Sea (56°12´N, 16°24´E) southeast of the Swedish mainland (Figure 1). Japanese mist nets and Helgoland traps were used for capture as previously described ( 5 ), and with the approval of the Swedish Museum of Natural History, Stockholm. Birds were trapped from March 17 to May 30, and from July 7 to November 13 of 2001, periods that are representative of spring and fall migrations, respectively. Trapped birds were banded and examined daily for ticks during these periods, except on April 2, September 17, 18, 22, and 24, and November 14, 16, and 17. Recaptured birds were not studied. Ticks attached to a bird's head were removed and, after species and stage identification, stored individually at -70°C. Figure 1 Scandinavian Peninsula in northern Europe. Location of Ottenby Bird Observatory (solid circle) on the southern tip of Öland Island in the Baltic Sea and nearby Blekinge County (shaded area) in mainland southern Sweden are shown in the inset. Directions of bird migration northward from outside northern Europe in the spring and back from Scandinavia and western Russia in the fall are shown by large arrows. DNA Extraction and Quantitative Real-Time PCR Tick DNA was extracted by using the Puregene DNA isolation protocol (Gentra Systems, Minneapolis, MN, USA) and stored at -20°C. DNA extracts were assayed for LB and relapsing fever (RF) group Borrelia by using a quantitative real-time polymerase chain reaction (qPCR) assay with probes and primers specific for the 16S rRNA gene ( 11 ). Serially diluted B. burgdorferi B31 and B. hermsii HS1 DNA were used as standards ( 11 ). Identifying and Genotyping Borrelia Species Borrelia species were identified by direct sequencing of the amplicons generated from the rrs (16S)-rrl (23S) intergenic spacer (IGS) or 16S gene PCRs ( 24 , 25 ). When necessary, nested modification of these assays was used to increase success of amplification. In addition, we obtained rrs-rrl IGS sequences of B. garinii isolated from skin biopsy specimens of erythema migrans lesions from 11 LB patients from southern Sweden ( 26 ). Positions with at least 2 different character states in >2 sequences each were considered polymorphic and included in the typing matrix. Sequences of new B. garinii IGS variants were deposited in GenBank database under accession nos. DQ307372–DQ307377. Statistical Analyses We used simple regression analysis, nonparametric Mann-Whitney U test, and standard parametric statistics giving the mean ± 95% confidence intervals (CIs) for continuous variables. We also used Fisher exact test, χ2 goodness of fit, and odds ratio (OR) procedures for proportions. Statistical analyses were conducted with StatView version 5.0.1 (SAS Institute Inc., Cary, NC, USA) and StatXact version 6 (Cytel Software, Cambridge, MA, USA). Results Tick Infestation of Birds According to the Ornithological Council's list of avian orders (available at http://www.nmnh.si.edu/BIRDNET/ORDERS/), 13,123 birds captured in this study were passerines (Passeriformes) (Table 1). In addition, there were 83 great spotted woodpeckers (Piciformes) and 54 sparrowhawks (Falconiformes). All studied birds were migratory. The 38 bird species studied comprised 6 ecologic guilds ( 27 ), each defined by a bird's foraging behavior. Three guilds comprised 19 species of ground-foraging birds and included 4,614 invertebrate feeders, 906 granivores, and 125 insectivores. In addition, 500 wrens and 30 marsh warblers, which are herbaceous plant–foraging insectivores that predominantly feed on the ground, were included in this group. The remaining 3 guilds and 17 species, referred to as other birds, comprised 223 raptors, 6,612 arboreal insectivores, and 250 other reed-foraging insectivores. Table 1 Infestation of migratory birds by Ixodes ricinus ticks and tick infection with Lyme borreliosis group spirochetes, Ottenby Bird Observatory study, Sweden, 2001 Bird species* No. birds No. ticks No. (%) birds infested Mean no. ticks/ infested bird No. (%) birds with infected ticks No. larvae No. (%) positive larvae No. nymphs No. (%) positive nymphs Ground foraging Erithacus rubecula 3,939 446 185 (5) 2.4 20 (11) 296 6 (2) 150 15 (10) Luscina luscinia 32 9 4 (13) 2.3 2 (50) 5 0 4 2 (50) Luscina svecica 85 8 5 (6) 1.6 1 (20) 0 0 8 1 (13) Turdus philomelus 261 141 24 (9) 5.9 10 (42) 88 14 (16) 53 17 (32) Turdus iliacus 51 22 9 (18) 2.4 2 (22) 5 0 17 4 (24) Turdus merula 193 170 44 (23) 3.9 15 (34) 36 11 (31) 134 28 (21) Turdus pilaris 23 6 3 (13) 2 1 (33) 3 1 (33) 3 1 (33) Sturnus vulgaris 30 18 9 (30) 2 2 (22) 7 3 (43) 11 4 (36) Prunella modularis 64 9 4 (6) 2.3 1 (25) 2 0 7 1 (14) Anthus trivialis 61 29 11 (18) 2.6 6 (55) 17 8 (47) 12 6 (50) Aluada arvensis 1 6 1 (100) 6 1 (100) 6 1 (17) 0 0 Fringilla coelebs 122 9 2 (2) 4.5 1 (50) 8 8 (100) 1 0 Carduelis flammea 441 1 1 (0.2) 1 0 1 0 0 0 Carduelis spinus 79 1 1 (1) 1 0 0 0 1 0 Pyrrhula pyrrhula 55 8 5 (9) 1.6 2 (40) 1 0 7 2 (29) Carduelis chloris 73 5 5 (7) 1 0 1 0 4 0 Carduelis cannabina 26 1 1 (4) 1 0 0 0 1 0 Carpodacus erythrinus 55 1 1 (2) 1 0 0 0 1 0 Emberiza schoeniclus 54 1 1 (2) 1 0 1 0 0 0 Troglodytes troglodytes 500 33 17 (3) 1.9 0 25 0 8 0 Acrocephalus palustris 30 1 1 (3) 1 0 0 0 1 0 Other Accipiter nisus 54 2 1 (2) 2 0 0 0 2 0 Lanius collurio 169 7 2 (1) 3.5 0 4 0 3 0 Dendrocopus major 83 8 1 (1) 8 1 (100) 2 0 6 4 (67) Hippolais icterina 87 15 2 (2) 7.5 0 15 0 0 0 Sylvia atricapilla 170 8 7 (4) 1.1 0 4 0 4 0 Sylvia borin 194 1 1 (0.5) 1 0 0 0 1 0 Sylvia curruca 621 11 8 (1) 1.4 2 (25) 4 0 7 2 (29) Sylvia nisoria 13 4 3 (23) 1.3 0 1 0 3 0 Phylloscopus sibilatrix 65 1 1 (2) 1 0 1 0 0 0 Phylloscopus trochilus 2,116 21 19 (1) 1.1 1 (5) 9 0 12 1 (8) Regulus regulus 2,212 1 1 (0.1) 1 0 0 0 1 0 Parus major 132 35 19 (14) 1.8 9 (47) 22 6 (27) 13 5 (39) Parus caeruleus 541 9 6 (1) 1.5 0 1 0 8 0 Certhia familiaris 37 1 1 (3) 1 0 0 0 1 0 Phoenicurus phoenicurus 341 23 12 (4) 1.9 2 (17) 12 0 11 2 (18) Sylvia communis 220 47 18 (8) 2.6 3 (17) 29 3 (10) 18 4 (22) Acrocephalus scirpaceus 30 1 1 (3) 1 0 0 0 1 0 Total 13,260 1,120 437 (3) 2.6 82 (19) 606 61 (10) 514 99 (19) *Ground-foraging species include invertebrate feeders (Erithacus rubecula through Sturnus vulgaris), insectivores (Prunella modularis and Anthus trivialis), granivores (Aluada arvensis through Emberiza schoeniclus), and herbaceous plant–foraging insectivores (Troglodytes troglodytes and Acrocephalus palustris). Other species include raptors (Accipiter nisus and Lanius collurio), arboreal insectivores (Dendrocopus major through Phoenicurus phoenicurus), and reed-foraging insectivores (Sylvia communis and Acrocephalus scirpaceus). The common names of the 38 bird species listed (from top to bottom) are European robin, thrush nightingale, bluethroat, song thrush, redwing thrush, blackbird, fieldfare, starling, dunnock, tree pipit, skylark, chaffinch, redpoll, siskin, bull finch, green finch, linnet, scarlet rosefinch, reed bunting, wren, marsh warbler, sparrow hawk, red-backed shrike, great spotted woodpecker, icterine warbler, blackcap, garden warbler, lesser whitethroat, barred warbler, wood warbler, willow warbler, goldcrest, great tit, blue tit, tree creeper, redstart, whitethroat, and reed warbler. We measured bird infestation with ticks and then compared the occurrence of the ticks on the birds with different foraging habits. Overall, 1,127 ticks were removed from 437 (3.3%) of 13,260 birds (Table 1). Of these ticks, 606 (54%) were larvae, 514 (46%) were nymphs, and 7 (0.6%) were adults of Ixodes ricinus, confirming that subadult ticks predominate on birds ( 5 ). (Because of their low number, the adult ticks, as well as 4 I. lividus nymphs removed from 1 bird, were excluded from further analyses.). I. ricinus larvae and nymphs were found on 226 (52%) and 310 (71%) of 437 infested birds, respectively; 99 (23%) of these birds were infested with both stages. The proportion of birds infested with larvae was higher in fall than in spring: 188 (2.1%) of 9,145 birds versus 38 (0.9%) of 4,115 birds (OR 2.3, 95% CI 1.6–3.2). In contrast, the proportion of birds infested with nymphs was similar between the 2 collection periods: 212 (2.3%) birds in fall and 98 (2.4%) in spring (OR 1.0, CI 0.8–1.2). The counts of captured birds with no ticks or >1 subadult tick followed a negative binomial distribution and are shown in Figure 2. The counts of these ticks on infested birds more specifically corresponded to a Zipf distribution (Kolmogorov-Smirnov statistic 0.05, p = 0.3; inset in Figure 2). Aggregation of infestation risk was further indicated by the finding that once a bird is infested with 1 subadult tick, the likelihood of infestation with >2 such ticks was higher than expected from a Poisson distribution (p 1 infected larva among the birds infested with >2 larvae (multiply infested). If the spirochetes were acquired transovarially, these indicators would not be expected to differ between the 2 groups. Conversely, a higher prevalence of infection in ticks from multiply infested birds in comparison to ticks from singly infested birds would be evidence of transmission from birds. Consistent with the latter hypothetical outcome, the proportions in singly infested and multiply infested birds were 7 (5.5%) of 128 and 21 (21.4%) of 98, respectively (OR 4.7, 95% CI 1.9–11.6). In another approach with multiply infested birds, we compared the count of infected larvae expected at 5.5% prevalence of infection (as found for the larvae of singly infested birds) with that observed in the larvae after the first positive larva has been identified. The observed and expected count of positive larvae was 33 and 6, respectively (p = 0.004), which is additional evidence of transmission of spirochetes from birds to larvae. Excluding the 1 skylark in the study, infestation by infected ticks was higher (3.0%) in 20 ground-foraging species than in 17 other species (0.6%) (p 0.5) (Figure 5). Figure 5 Relationship between Lyme borreliosis spirochete load and proportion of infected larvae (A) and nymphs (B). Values 0.4). To validate this result, which suggests that migratory passerines transmit LB spirochetes to ticks with similar efficiency, we compared LB spirochete counts in the larvae from the 2 bird groups. The cell counts were available for 52 larvae from 25 ground foragers and 9 larvae from 5 birds of other species. Weighted means of spirochetes per infected larvae from ground-foraging birds and other bird species were 135 (95% CI 21–862) and 23 (95% CI 2–318), respectively (p = 0.4). This was additional evidence that the 2 bird groups were equally competent in transmitting infection to larvae. Discussion This was the first large-scale study to show that migratory passerine birds participate in the enzootic maintenance of Borrelia spirochetes, including species and genotypes associated with LB in humans. By combining 2 approaches, quantification of infection in vector ticks and molecular typing, we demonstrate that these birds constitute an epidemiologically important alternative reservoir of LB, as well as a means for wide distribution of the pathogen. This study's approach of characterizing Borrelia infection of ticks engorged on birds is analogous to xenodiagnosis, which is commonly used in assessing reservoir competence in the laboratory ( 17 ). A correlation between rate of tick infestation and infestation with infected ticks is evidence of a bird source of infection. Consistent with this source, the proportion of birds infested with multiply infected larvae and the observed counts of infected larvae on individual birds exceeded the baseline values assumed to represent a hypothetical transovarial transmission. Furthermore, infection prevalence correlated with the number of spirochetes in larvae, which suggests a new variable for quantifying reservoir competence for Borrelia transmission. Finally, Borrelia species composition in larvae, namely, predominance of B. garinii and absence of B. afzelii, indicates the bird source of infection ( 33 ). However, inferring reservoir competence from measuring infection of naturally infesting ticks has drawbacks. Collection of only birds that had ticks on them at the time of capture could lead to an underestimation of the prevalence of infection among the studied bird population. Also, in this study we could not follow-up and quantify the infection of the nymphs that emerge from infected larvae, a transition that determines the ability of the nymphs to infect other hosts during subsequent feeding ( 17 ). A negative binomial distribution of natural loads of subadult I. ricinus on migratory birds is a common characteristic of ectoparasitism ( 34 , 35 ), including infestation with ticks ( 36 ). Similar to other hosts, infestation of migratory birds is nonrandom, presumably due to different tick densities at stopover sites along the migration routes. These routes likely run in a south–north direction and within boundaries of central and northeastern Europe. Two indications of this are infestation of birds almost exclusively with I. ricinus ticks, which prevail in these regions, and the absence of I. persulcatus, a common bird parasite in eastern Europe and Asia ( 37 ). Different activation times of larvae and nymphs along this geoclimatic axis also determine the dissociation between infestations with the 2 stages, as indicated by lack of correlation between their numbers on a given bird, as well as relatively infrequent co-infestations. This dissociation is further supported by the evidence of distinct histories of infection with LB spirochetes of larvae and nymphs: 1) greater prevalence of infection in nymphs than in larvae; 2) correlation between prevalence of infection and spirochete counts in larvae, but not nymphs; and 3) bimodal distribution of spirochete counts in nymphs, but not larvae, presumably due to residual infection in the nymphs acquired during feeding at larval stage. Thus, the 2 subadult tick stages represent different aspects of migratory birds' involvement in the maintenance of Borrelia. Whereas both stages contribute to the assessment of geographic dissemination and carrying capacity of infected vector ticks by birds, larvae provide a direct measure of birds' competence in transmitting the spirochetes. In comparison with other hosts, birds appear to be infested with fewer ticks ( 19 , 22 , 38 ). For example, the 2.1–2.6 ticks per infested bird density found in this study is ≈20–30 times less than that found on rodents in south-central Sweden ( 39 ). Conversely, migratory bird population estimates suggest that their actual contribution in hosting, infecting, and disseminating ticks may be at least as important as that of other hosts. For example, ≈150 million migratory passerine birds come to their breeding grounds in Sweden in the spring ( 40 ), and at least 2 times that number migrate in the fall. Assuming that our findings are representative of these bird populations and at observed infestation and infection rates, ≈15 million infested birds would disseminate 40 million ticks, of which 5.6 million would be infected with LB group spirochetes. Five million of these ticks would carry B. garinii, and at least one third would be infected by birds. The 16% extrapolated prevalence of B. garinii found in nymphs feeding on migratory passerines in this study corresponds to ≈50% of that found in pheasants in the United Kingdom, where these birds are the major reservoir of this spirochete ( 13 ). Thus, migratory passerines contribute to influx of B. garinii into the natural circulation, where this species is known to adapt to local enzootic transmission cycle involving mammals. Measuring the occurrence of ticks in 2 uniquely large migratory bird collections in Scandinavia at a 10-year interval provided consistent evidence of greater risk for exposure to ticks among ground-foraging birds. As a result of this increased risk, the infestation rate with infected ticks and the proportion of presumably infected birds were greater in ground feeders than in other birds. However, the transmission of spirochetes from bird to tick, defined as the amount and prevalence of infection in ticks, was similar between the 2 migratory bird groups. Thus, a bird's feeding behavior, rather than other biologic differences, is a critical determinant of its reservoir potential. Notwithstanding exceptions and as a group, those birds that spend time on the ground contribute most effectively to the maintenance of both the vector ticks and the spirochetes. The agent of LB in North America, B. burgdorferi, is associated with different vertebrate reservoirs, including birds ( 4 , 31 ). The American robin, an abundant and commonly tick-infested passerine, is as effective as mice in reservoir competence for this bacterium ( 17 ). Understanding the contribution of this and other alternative reservoirs in enzootic maintenance of B. burgdorferi is prerequisite for advancing prevention strategies for LB ( 11 ).