- Record: found
- Abstract: found
- Article: not found
The anti-HCV, Sofosbuvir, versus the anti-EBOV Remdesivir against SARS-CoV-2 RNA dependent RNA polymerase in silico
Read this article at
Abstract
Abstract
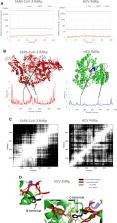
Coronavirus diseases 2019 (COVID-19) are seriously affecting human health all over the world. Nucleotide inhibitors have promising results in terms of its efficacy against different viral polymerases. In this study, detailed molecular docking and dynamics simulations are used to evaluate the binding affinity of a clinically approved drug, sofosbuvir, with the solved structure of the viral protein RNA-dependent RNA polymerase (RdRp) and compare it to the clinically approved drug, Remdesivir. These drugs are docked onto the three-dimensional structure of the nsp12 protein of SARS-CoV-2, which controls the polymerization process. Hence, it is considered one of the primary therapeutic targets for coronaviruses. Sofosbuvir is a drug that is currently used for HCV treatment; therefore, HCV RdRp is used as a positive control protein target. The protein dynamics are simulated for 100 ns, while the binding is tested during different dynamics states of the SARS-CoV-2 RdRp. Additionally, the drug-protein complexes are further simulated for 20 ns to explore the binding mechanism. The interaction of SARS-CoV-2 RdRp as a target with the active form of sofosbuvir as a ligand demonstrates binding effectiveness. One of the FDA-approved antiviral drugs, such as sofosbuvir, can help us in this mission, aiming to limit the danger of COVID-19. Sofosbuvir was found to bind nsp12 with comparable binding energies to that of Remdesivir, which has been reported for its potential against COVID-19 RdRp and is currently approved by the FDA.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Clinical Characteristics of Coronavirus Disease 2019 in China
- Record: found
- Abstract: found
- Article: not found
UCSF Chimera--a visualization system for exploratory research and analysis.
- Record: found
- Abstract: found
- Article: not found

