- Record: found
- Abstract: found
- Article: found
A prognostic model for platinum‐doublet as second‐line chemotherapy in advanced non‐small‐cell lung cancer patients
Read this article at
Abstract
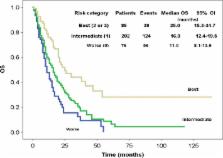
Poor prognosis of advanced non‐small‐cell lung cancer ( NSCLC) patients and the promising therapeutic effect of platinum urge the oncologists to evaluate the role of platinum doublet as second‐line chemotherapy and establish the definition of platinum sensitivity in NSCLC. We retrospectively analyzed 364 advanced NSCLC patients who received platinum‐doublet regimens as second‐line chemotherapy after platinum‐based first‐line treatment. Patients were divided into four groups by their time‐to‐progression ( TTP) after first‐line chemotherapy: 0–3, 4–6, 7–12, and >12‐month group, respectively. Treatment efficacy of patients' overall survival ( OS), progression‐free survival ( PFS), and response rate ( RR), as well as treatment‐related toxicity, were compared among the four groups. A prognosis score system and a nomogram were established by Cox proportional hazard model, and validated by concordance index (c‐index). Median OS was 14.0, 16.0, 20.0, 25.0 months for patients in the 0–3, 4–6, 7–12, >12‐month group, respectively. Age ≤60 years ( P = 0.002), female ( P = 0.019), and TTP>12 months ( P = 0.003) were independent prognostic factors. Prognostic score was calculated by adding 1 point each for any of the above three indicators, with a c‐index of 0.590 (95% confidential interval [ CI], 0.552–0.627). Median OS were equal to 25.0, 16.0, and 11.0 months for best (2–3 points), intermediate (1 point) and worst (0 point) category, respectively ( P < 0.0001). A nomogram that integrated patient's age, gender, and TTP for OS has a c‐index of 0.623 (95% CI, 0.603–0.643). Female, younger than 60 years, and TTP greater than 12 months may indicate prolonged survival after platinum‐doublet second‐line chemotherapy in advanced NSCLCpatients.
Related collections
Most cited references11
- Record: found
- Abstract: found
- Article: not found
Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial.
- Record: found
- Abstract: found
- Article: not found
Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer.
- Record: found
- Abstract: found
- Article: not found