- Record: found
- Abstract: found
- Article: found
Biocompatibility and Bioactivity of Set Direct Pulp Capping Materials on Human Dental Pulp Stem Cells
Read this article at
Abstract
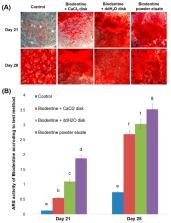
In this study, we assessed the biocompatibility and bioactivity of various pulp capping materials—ProRoot MTA (Dentsply Tulsa Dental Specialties), Biodentine (Septodont), TheraCal LC (Bisco), and Dycal (Dentsply Caulk)—on human dental pulp stem cells (hDPSCs). Experimental disks (diameter, 7 mm; height, 4 mm) were stored in a humified incubator at 37 °C for 48 h. Then, the pulp capping materials were tested for cytotoxic effects by methyl-thiazoldiphenyl-tetrazolium and scratch wound healing assays, and for mineralization potential by Alizarin red S (ARS) staining assay and alkaline phosphatase enzyme (ALP) activity. Cell viability and cell migration did not significantly differ between ProRoot MTA, Biodentine, and control ( p > 0.05). TheraCal LC exhibited slower cell migration on days 2–4 compared to control ( p < 0.05), and Dycal showed no cell migration. ALP activity was highest with Biodentine on days 10 and 14, and was lowered with TheraCal LC and Dycal ( p < 0.05). In the ARS assay, hDPSCs grown in ProRoot MTA and TheraCal LC eluates showed significantly increased mineralized nodule formation on day 21 compared to Biodentine, Dycal, and control ( p < 0.05). These findings indicate that ProRoot MTA, Biodentine, and TheraCal LC exhibit better biocompatibility and bioactivity than Dycal.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Mineral trioxide aggregate: a comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action.
- Record: found
- Abstract: found
- Article: not found
Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations.
- Record: found
- Abstract: found
- Article: not found