- Record: found
- Abstract: found
- Article: found
Mapping the IκB Kinase β (IKKβ)-binding Interface of the B14 Protein, a Vaccinia Virus Inhibitor of IKKβ-mediated Activation of Nuclear Factor κB*
Abstract
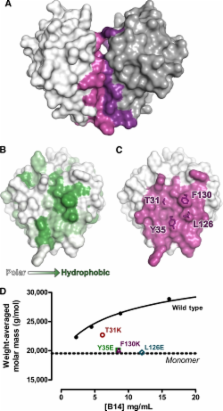
The IκB kinase (IKK) complex regulates activation of NF-κB, a critical transcription factor in mediating inflammatory and immune responses. Not surprisingly, therefore, many viruses seek to inhibit NF-κB activation. The vaccinia virus B14 protein contributes to virus virulence by binding to the IKKβ subunit of the IKK complex and preventing NF-κB activation in response to pro-inflammatory stimuli. Previous crystallographic studies showed that the B14 protein has a Bcl-2-like fold and forms homodimers in the crystal. However, multi-angle light scattering indicated that B14 is in monomer-dimer equilibrium in solution. This transient self-association suggested that the hydrophobic dimerization interface of B14 might also mediate its interaction with IKKβ, and this was investigated by introducing amino acid substitutions on the dimer interface. One mutant (Y35E) was entirely monomeric but still co-immunoprecipitated with IKKβ and blocked both NF-κB nuclear translocation and NF-κB-dependent gene expression. Therefore, B14 homodimerization is nonessential for binding and inhibition of IKKβ. In contrast, a second monomeric mutant (F130K) neither bound IKKβ nor inhibited NF-κB-dependent gene expression, demonstrating that this residue is required for the B14-IKKβ interaction. Thus, the dimerization and IKKβ-binding interfaces overlap and lie on a surface used for protein-protein interactions in many viral and cellular Bcl-2-like proteins.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Inference of macromolecular assemblies from crystalline state.
- Record: found
- Abstract: found
- Article: not found
Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures.
- Record: found
- Abstract: found
- Article: not found